2023-06-25 Hits(403)
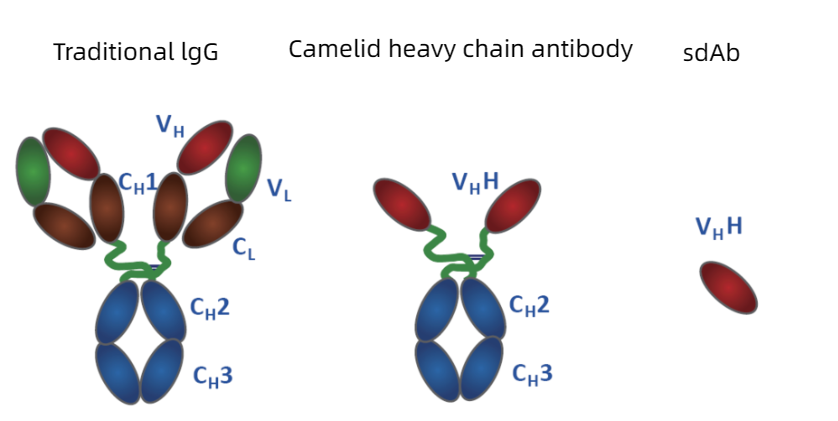
Nanobody was first proposed and reported in Nature by Belgian scientists in 1993. The traditional antibody molecule (IgG) is composed of two identical heavy chains and light chains. The light chain of antibody molecule contains one VL region and one CL region, while the heavy chain has one VH region and three CH regions (CH1, CH2 and CH3).
Alpaca only has heavy chain antibody but lacks light chain, which is composed of four HC's and two VH's. Among them, VHH region is also called nano antibody or single domain antibody (sdAb) and Nanobody (Nb). It’s crystal is 2.5nm, 4nm in length and only 15KDa in molecular weight. Nano-antibodies are mainly used in biomedical research and development (genetic engineering drug research and development, ADC drug research and development); Used for clinical diagnosis in vitro (colloidal gold method, enzyme-linked immunosorbent assay, electrochemiluminescence method); It is used in basic research fields such as tumor research and immunology. At present, the development of CAR-T therapeutic antibody has attracted more and more researchers' attention.
(1) stability at high temperature and pH;
(2)VHH can recognize antigen sites that are not usually recognized by conventional antibodies;
(3) Their small molecular fragments are helpful for rapid tissue penetration and labeling applications, including crossing the blood-brain barrier;
(4) Cost-saving substitutes for mass production.
Nanobody (VHH antibody) has a small molecular weight of about 15kDa, which is equivalent to the variable region of the heavy chain or light chain of traditional IgG antibody. Therefore, the secondary antibody against VHH antibody is not universally applicable, and it is necessary to co-express the VHH fusion Fc fragment to solve the problem of secondary antibody recognition (KMD Bioscience provides a variety of anti-alpaca secondary antibodies, which can be used to detect the titer of immune serum, cat. #: SPAB01, SPAB03).
Based on the special structure and properties of Nanobody, it is favored by people in the field of CAR-T therapeutic antibody development.
CAR-T therapy is a new type of targeted therapy for tumor, that is, chimeric antigen receptor T cell immunotherapy. By means of genetic engineering technology, activated T cells are equipped with a positioning navigation device CAR (tumor chimeric antigen receptor) to turn T cells into "super soldiers", that is, CAR-T cells. With its positioning navigation device CAR, tumor cells in vivo can be accurately and specifically identified and eliminated.
KMD Bioscience can provide a variety of camel-derived Nanobody development services, including alpacas, llamas, camelus, etc. Based on the phage display technology platform, KMD Bioscience can provide major experimental links including antigen design, alpaca immunity, library construction and screening, and activity function identification, and provide alpaca VHH antibodies with high specificity and high affinity for scientists around the world.
It mainly includes the following steps: alpaca immunization, phage library construction, antibody library screening, antibody expression and function identification. The detailed process is as follows:
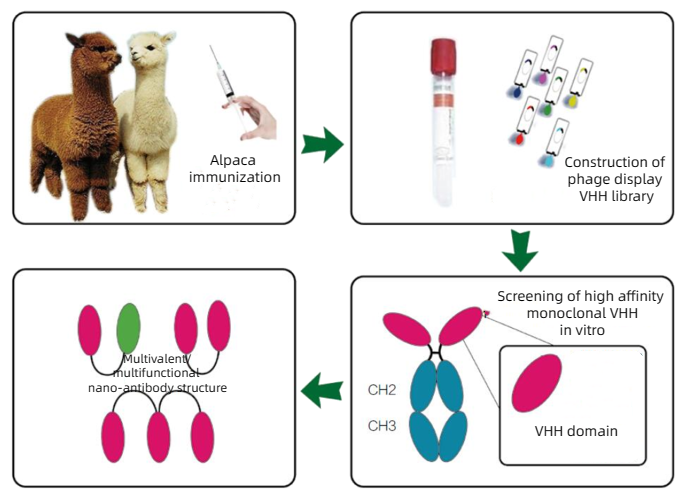
KMD Bioscience provides healthy adult alpacas (28-36 months old) for immunization, and high-quality libraries and monoclonal antibody cell lines can be obtained in about 16-20 weeks, and complete experimental process records and immune dynamic videos can be delivered. According to the antigen type of customers, KMD Bioscience will make specific immune antigen generation plans and immunization plans.
5.1.1 Generation of Antigen: The customer provides the antigen, the dosage of Freund's adjuvant is 1ml, and the antigen and adjuvant are mixed in a ratio of 1:1 for immunization.
5.1.2 Alpaca Immunization: the alpaca was immunized for 5 times with an interval of 20 days, and the alpaca was injected in the neck, and the alpaca was observed for discomfort symptoms 1 hour after immunization.
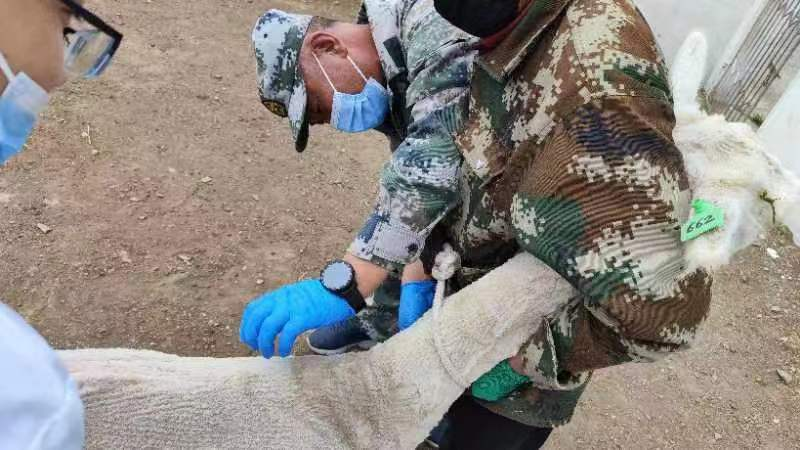
5.1.3 Blood Collection: Blood was collected before immunization, and kept as a negative control. After the third and fourth immunization, 5ml titer of blood was measured at intervals of one week, and the serum titer was detected by ELISA, and the titer met the requirements > 105. KMD Bioscience provides customers with a clear information table of immunization time nodes, as shown in Table 1:
Table 1: Example of immune time course of generation of nanobody
|
Times |
Immunization Date |
|
first time |
November 25th, 2020 |
|
second time |
December 16th, 2020 |
|
third time |
January 6th, 2021 |
|
fourth time |
January 27th, 2021 |
|
fifth time |
February 18th, 2021 |
5.1.4 PBMC cell separation: take 100-120mL of peripheral blood, and the total number of PBMC cells is 4*107-108, all of which will be mailed to customers.
KMD Bioscience displayed VHH antibody (Nanobody) with pIII protein of M13 phage, prepared TG1 transduced competent cells, constructed phage vector, and measured phage storage capacity.
5.2.2CDNA Reverse Transcription Synthesis: prepare cDNA according to the operation of commercial kit.
5.2.3PCR Amplification: Using cDNA as template, the variable region of heavy chain antibody gene was amplified by PCR for 2 rounds.
5.2.4 Connecting Vectors and Conducting Electric Transformation: enzyme digestion, connecting phagemid vectors, transforming TG1 host bacteria by electric shock, and constructing antibody library.
5.2.5 Establishment of Bacterial Library/Phage Library: Calculate the library capacity, and the library capacity delivery standard is 108 ~ 109.
The commonly used screening methods are solid phase screening, magnetic beads and cell screening (commonly used for antibody screening of GPCR and ion channel receptors).
KMD Bioscience provides customized screening system for customers. According to different needs of customers, we provide solid phase screening, solid phase/liquid phase screening and cell screening methods. The first two are suitable for screening target antigens such as proteins and peptides; The third is suitable for antibody screening of GPCR, ion channel receptor. Through different screening methods, different types of blocking antibodies can be efficiently screened for customers.
5.4.1 Expression: Mammalian expression system was constructed for antibody expression. KMD Bioscience established a perfect mammalian expression system, including but not limited to cell lines such as FreeStyle 293-F cell line and Expi CHO-S, and cooperated with high expression vector designed by KMD Bioscience (containing full-length CMV promoter and optimized secretory signal peptide sequence) for recombinant antibody expression and generation.
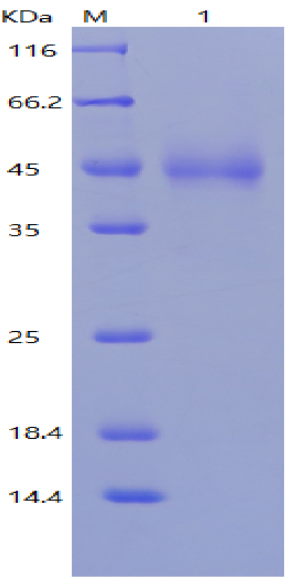
5.4.2 Purification: the Nanobody is purified by nickel column.
5.4.3 Affinity Determination: The purified Nanobody is tested for Biacore affinity.
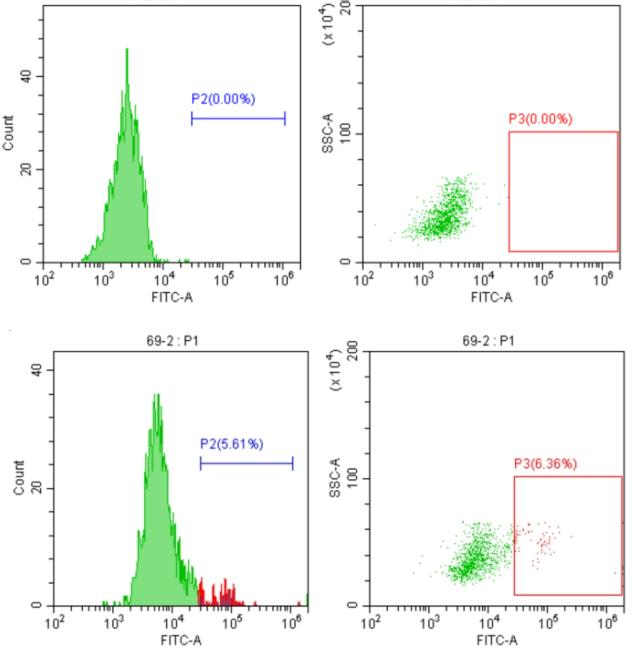
KMD Bioscience has a large alpaca breeding base in northwest China. The company provides 200+ alpaca Nanobody generation services for scientific research users every year, and the generation cycle is short. It takes about 16-20 weeks to obtain high-quality library and monoclonal antibody cell lines, and can deliver 2ml VHH antibody library with a library capacity of 108-109. Welcome scientific research users to contact us.