Service Line:+86-022-82164980
Address:FL-4, Building A5, International Enterprise Community, Tianjin, China
Email:[email protected]

Introduction to scFv Antibody Phage Display Platform
|
scFv, the most commonly used recombinant antibody, is approximately 25 kDa in size and consists of a heavy chain (VH) and a light chain (VL) connected by a short flexible peptide. With its small size and high specificity, scFv can be large-scale produced in prokaryotic expression systems and is widely used for the diagnosis and treatment of viral, bacterial, toxin, and tumor antigens. KMD Bioscience has established a solid platform for scFv preparation with a well-established laboratory system for generating scFv antibodies derived from naïve or immunized mouse and rabbit spleen cells, hybridoma cells, and human B lymphocytes. We meet the different needs of customers, provide high-quality scFv library construction and other efficient scientific research technology services, saving experimental costs and time for customers, and contributing wisdom and strength to scientific research.
|
|
The Procedure of scFv Antibody Phage Display Construction Service
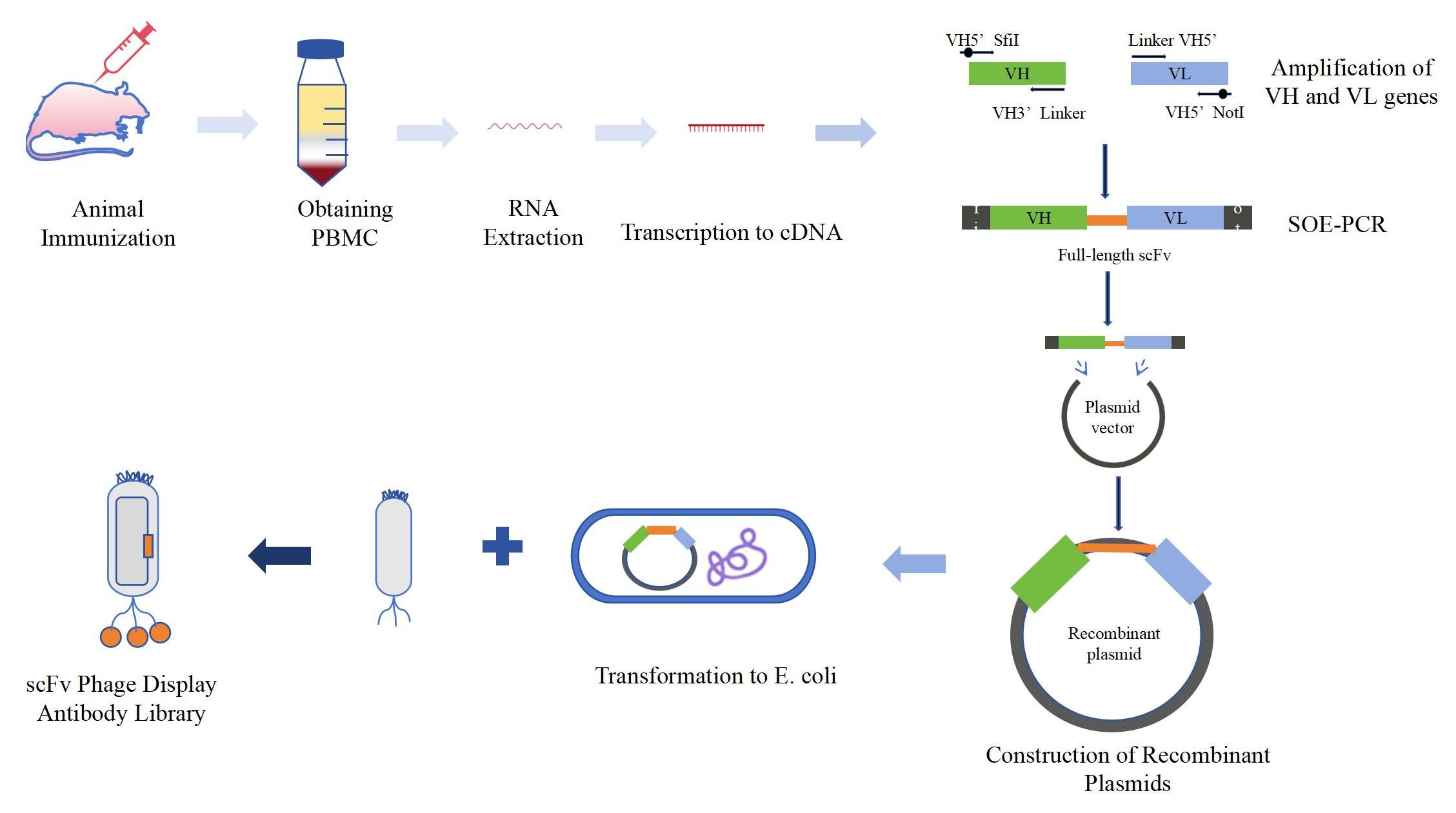
Related Services of scFv Antibody Phage Display
We combine our mature recombinant antibody expression platform with hybridoma technology and complete antibody sequencing technology to generate highly specific scFv to meet our customers' needs. In addition, we provide further scFv antibody library screening, scFv antibody expression and purification, functional studies, and antibody optimization so that they can be used in the next step of research.
|
|
|
Recommended Special Services
KMD Bioscience can provide customers with antibody related services, and its technology platform provides customers with higher quality services.
 |
 |
 |
|
|
|
|
scFv Antibody Phage Display Related Technical Service Resources
We are willing to share with you the rich technology and resources used in scFv antibody phage display related technical services to solve your doubts. If you need to view more, please refer to our related articles.
|
|
|
|
|
|
|
|
FAQ-scFv Antibody Phage Display
1. What are the scFv antibodies?
A: Single-chain antibodies are mainly produced by hybridoma, murine spleen, and human B cells. The scFv (single-stranded fragment variable region) is a non-covalent heterodimer composed of VH and VL domains, which can be used to construct recombinant scFv (single-stranded fragment variable region) antibodies. The scFv (single-chain Fv) is modified by the variable region of traditional antibodies. The scFv acts as a small-molecule antibody. Compared with the complete conventional antibody, the scFv molecular weight is only about 1/6 that of the complete whole antibody, which makes the scFv more penetrating and less immunogenic. The Fv fragment is the smallest unit of immunoglobulin molecules with antigen-binding activity. Antibodies in the form of scFv (single chain variable fragment) consist of variable regions of heavy chain (VH) and light chain (VL) that are linked together by a flexible peptide adaptor that can be expressed as a single chain variable region antibody (single chain fragment variable, scFv) in E.coli, enabling protein engineering to improve scFv properties, such as increased affinity and altered specificity. KMD Bioscience has many years of experience in tailoring recombinant antibodies, providing a variety of different methods for tailoring different antibodies and selecting antibodies for customers according to their specific research needs. At the same time, KMD Bioscience provides scFv antibody production services, Fab antibody customized services other antibody customized services, and antibody humanization services. KMD Bioscience also provides whole-chain services including antigen preparation, scfv antibody design, phage library construction, and recombinant antibody purification.
2. What are the main aspects of the scFv antibody discovery service?
A: To obtain scFv, messenger ribonucleic acids were first isolated from hybridomas or spleen, B cells, and bone marrow and then reverse transcribed as a template for amplification. The heavy chain (VH) and light chain (VL) genes of the antibody were then separately amplified by RT-PCR. Using the heavy and light chain genes to form the scFv gene fragments we need, and then connecting them to the vector, using this method, the construction of a single-chain variable fragment gene library with different ranges can be completed. They are then transformed into different host cells (such as E. coli, yeast, etc.) to build libraries containing the target antibody fragments. Then, KMD Bioscience carried out repeated rounds of single-chain variable fragment phage screening and enrichment, and finally completed the construction of the scfv antibody library, the target scFv antibody fragment of the client was selected from the constructed antibody library, and then the scfv antibody sequence was sequenced and analyzed to confirm its sequence and characteristics. KMD Bioscience purified the antibodies according to the customer's request and then delivered them to the customer. At the same time, KMD Bioscience can also provide scFv antibody humanization services, antigen preparation, recombinant antibody purification, and other whole-chain services.
3. Advantages of the scFv antibody discovery services?
A: scFv antibodies have many advantages compared with mAbs. First, stuff antibodies are highly stable and can be stored for several years at 4℃. Meanwhile, the genes of scfv antibodies are easy to manipulate, and scfv antibodies can bypass hybridoma and immunity to produce. Furthermore, higher affinity mutants of scFv can be generated by site-directed mutagenesis, which is easier and simpler to proceed. In contrast to soluble singles, singles from phage display technology can be used directly during mouse immunization to produce anti-idiotypic antibodies without adjuvants because phage particles are also good immunogens. The scFv is the minimal functional unit of the antibody and has shown promise in cancer radioimmunoscintigraphy due to its special properties, including higher tumor penetration and faster clearance compared to parent Ig. After scFv antibody design and antibody customization, the antibody can be displayed on the phage. The scFv single-chain antibodies play a major role in cancer therapy to develop targeting vectors for curable gene delivery. These viral vectors are known to be very effective in delivering therapeutic genes to target cell populations, however, their clinical use may be limited by eliciting an immune response. The scFv Sb can also be used as diagnostic reagents. In the past few years, antibodies customized by scFv antibodies have become candidates for many common no-reagents.
4. Structural specificity of scFv antibodies and the role of aptamers?
A: As a small molecule genetically engineered antibody, ScFv has a molecular weight of only about 1 / 6 that of the intact antibody, which makes ScFv more penetrating and less immunogenic. The Fv fragment is the smallest unit of the immunoglobulin molecules, and the Fv fragment has antigen-binding activity. Antibodies in the form of scFv (single chain variable fragment) consof variable regions of heavy chain (VH) and light chain (VL) that are linked together by a flexible peptide connector that expresses single chain variable region antibodies in E.coli, enabling protein engineering to improve scFv properties, such as increased affinity and altered specificity. The length of the flexible DNA aptamer connecting the two V domains in the scFv antibody is critical for the correct folding of the antibody. As analyzed, the scFv antibody peptide connector must span 3.5 nm (35 A) between the carboxyl terminus of the variable region and the amino end of another domain, and cannot have an effect on domain folding or interfere with the ability to form the intact antigen-binding site. In addition to the de novo adaptor peptide, we can also apply the peptide sequence derived from the protein structure to provide compatible length and conformation in the variable region of the bridging scFv (single-stranded fragment variable region) to stabilize the antibody spatial structure without change. In addition to the length of the joint, the amino acid composition of the adaptor also plays an important role. They must be hydrophilic, preventing the adaptor peptide from inserting within or between variable domains throughout the protein folding process.
.png)
Fig: The effect of the culture medium additive on the scFv production. (Reference documentation: Shi X, Karkut T, Chamankhah M, Alting-Mees M, Hemmingsen SM, Hegedus D. Optimal conditions for the expression of a single-chain antibody (scFv) gene in Pichia pastoris. Protein Expr Purif. 2003 Apr;28(2):321-30. )
5. In the scFv antibody discovery service, we often encounter low antibody affinity and poor stability. How can we solve it?
A: scFv antibody has low affinity compared to an intact antibody. At the same time, the scFv antibody is prone to degradation in the body and is not as stable as the intact antibody. KMD Bioscience optimizes the sequence of scFv antibodies through genetic engineering means, such as introducing some mutations and deleting unwanted irrelevant sequences, to help improve the scFv antibody affinity. At the same time, KMD Bioscience increases the affinity by building scFv polysomes (such as Diabodies and tribodies) to increase the binding valence between antibody and antigen. KMD Bioscience also humanizes scFv to reduce its immunogenicity by genetic means, while possibly increasing the affinity of antibodies. The selection of appropriate Linker can help increase the stability of scFv antibodies because the length and composition of Linker have important effects on scFv stability. Optimize the expression system: at the same time, the expression of the scFv antibody is not the same in different expression systems, and the expression amount may be too low. By trying to express scFv in the prokaryotic system (e.g., E. coli), yeast system, or mammalian cell system to find the most suitable condition for stable expression. We also added protective groups, such as PEG (polyethylene glycol), to improve the stability of the antibody within the body.