2023-08-30 Hits(244)
Once the inhibitory protein formed by lacI cells is fused with the lac manipulator, it cannot affect the transcription and expression of external genes, thus ensuring the healthy growth of the host cells.IPTG is also an intermediate substance that can be fused with lactose hydrolysis, which cannot be assimilated by the cells but can bind to the inhibitory protein so that the cells can control a large amount of transcription and high-efficiency expression of externally financed genes. massive transcription and efficient expression.
1. Increase protein solubility and folding: high temperature is easy to make the proteins expressed in the form of aggregates into inclusion bodies, so a low temperature can be chosen to induce expression.
2. Increase the translation level: adjust the distance between the SD sequence and AUG, change the base by point mutation, and increase the mRNA stability.
3. Reduce the metabolic load of the cell and increase the expression level: separate the growth of bacteria from the induced expression of exogenous genes; use chemical induction, temperature induction, etc.
4. Rare codon optimization: most amino acids have more than one codon, when the mRNA of a heterologous target gene is abnormally expressed, the number of tRNAs directly responds to codon preference, and the rarity or lack of one or more tRNAs leads to translation arrest.
1. Digest vector DNA and target gene with an appropriate restriction endonuclease.
2. Connect the target gene and vector according to the ligation procedure and transform them into transformed DH5α receptor cells.
3. Positive colonies were picked and added to 5 ml Lb (0.1 g/l ampicillin) and incubated at 37°C overnight.
4. Transfer to 2 ml LB ( 0.1 g/l ampicillin) at 2 % v/v ratio, and continue to incubate at 37℃ for 2.5 h to the bacterial logarithmic growth stage, add 0.1 mmol/l IPTG 2 µl to induce for 3-4 h. Meanwhile, set up induction without IPTG as a control.
5. Take 1 ml of the above bacterial solution, centrifuge at 12000 rpm for 10 min, and collect the bacterial precipitate.
6. Resuspend in 100 µl PBS on ice, and add PMSF to a final concentration of 10 mmol/l. Shake with a shaker to mix well, add 2× spiking buffer, boil for 10 min to denature, and centrifuge at 12,000 rpm for 10 min.
7. 10 µl of each sample before and after induction was analyzed by SDS-PAGE electrophoresis.
The speed of a charged particle traveling in an electric field is directly proportional to the strength of the electric field and the net charge of the charged particle, and inversely proportional to the radius of the particle (molecular weight and structure) and the viscosity of the medium. If the reagent is a mixed protein solution, the various proteins have different numbers of atoms at their isoelectric points, which creates different electron migration bands during electrophoresis.
(1) Preparation of gel: configure the separation gel, ddH2O 4.0ml, 30% reserve gel 3.3ml, 1.5M Tris-HCl 2.5ml, 10% SDS 0.1ml, 10% APS 0.1ml. 1ml of the above mixture, then seal the bottom with 10 ul of TEMED (N,N,N,N'N'-tetramethylene diamine), and then the remaining ul, mixed well, and then masked the top of the head with a glass plate filled with a twenty percent ethanol solution. Make sure the liquid level is flat. Concentrated gel was prepared with ddH2O 1.4 ml, 30% reserve gel 0.33 ml, 1M Tris-HCl 0.25 ml, 10% SDS 0.02 ml, 10% APS 0.02 ml, and TEMED 2 ul. After removing or separating the water within the gel, the mixture was re-poured, and then the grate was quickly placed into the glass to fully polymerize, taking about 15-30 min.
(2) Sample addition and electrophoresis: A certain amount of 2xSDS buffer was added to the sample, heated for 3-5 min, centrifuged at 12,000g for 1 min, and then the supernatant was taken for SDS-polyacrylamide gel electrophoresis. 10ul of induced and uninduced treated samples were added to the cuvette and protein markers were added to adjacent lanes. The electrophoresis buffer was injected into the electrophoresis tank and the power supply was turned on. The voltage of the concentration gel was about 80 V, while the voltage of the separation gel was 120 V. The electrophoresis was finished when the bromophenol blue went to the bottom.
(3) Staining and decolorization of proteins: Remove the gel from the glass plate and stain it with Caulmers Brilliant Blue staining solution at room temperature for 4-6 h. After staining, remove the gel from the staining solution and put it into the decolorizing solution and decolorize it several times until the protein bands are clear.
(4) Storage of film camera: the film that has been decolorized will be videotaped in the image processing mode, and the result will be stored in the computer, while the gel can be stored in double-distilled water.
|
Frequently Asked Questions |
Causes |
Solutions |
|
The strip drag tail |
The gel concentration is too large / the sample dissolution effect is not good |
Conple sample sample samples; electrophoresis buffer; reduce the gel concentration |
|
The middle concave is warped on both sides |
The middle of the gel solidified unevenly |
Operate after sufficient solidification |
|
The middle is convex and concave on both sides |
Air bubbles at the bottom of the plates |
Add appropriate buffer to remove air bubbles |
|
The strip is thick |
Not concentrated |
Increase the length; ensure the stable voltage and ensure the correct storage pH |
|
There is precipitation at the bottom of the sample hole |
Inactivation of reducing agents enables the polymerization of protein molecules into macromolecules |
Add an appropriate amount of DTT or β -mercaptoethanol; EDTA supplementation to prevent oxidation of the reducing agent |
|
Appearance of texture |
The sample had insoluble particles |
Centrifugation before solvent tropic / sample addition |
SDS-PAGE results before and after prokaryotic induced expression of a fusion protein of size 43KDa:
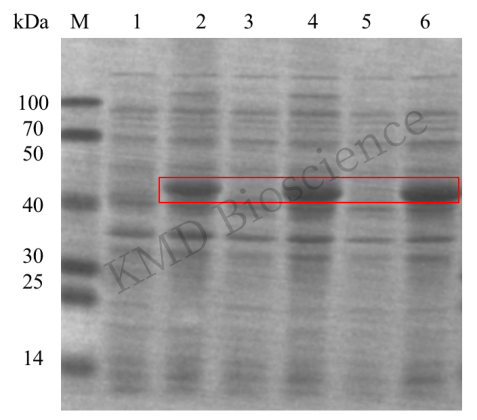
M: Protein Marker; lanes 1, 3, 5: 3 monoclonal strains before induction; lanes 2, 4, 6: 3 monoclonal strains after induction.
1. Clinical medicine: electrophoresis technology plays an important role in clinical diagnosis, providing a new means for detecting isoenzymes, various proteins and so on.
2. Biopharmaceuticals, drug screening and analysis: analyzing drugs and their metabolites in biological samples, analyzing drug impurities, analyzing important, etc.
3. Food safety and microbial identification: sample strips can react to the community similarity between different samples, and time-of-flight mass spectrometry, electrochemical detector, etc., to improve the reliability of trace detection.
4. Agriculture and animal husbandry production: It can be used for the identification of hybrid progeny, kinship analysis, genetic positioning and other aspects.
KMD Bioscience has abundant prokaryotic expression vectors and a variety of expression strains, through the accumulation of technology of more than 400 single prokaryotic protein preparation projects every year, customers only need to provide us with a piece of protein sequence, CDS or protein name, we will be able to design a complete protein expression and purification program in a relatively short period, to provide customers with high-quality protein products, to help your experimental progress.