2025-04-14 Hits(113)
Antibody-Drug Conjugate
Definition of Antibody-Drug Conjugate
Antibody-drug conjugate (ADC) is a targeted cancer therapy that combines the specificity of monoclonal antibodies with the potent cell-killing ability of cytotoxic drugs. ADCs are designed to deliver highly toxic drugs directly to cancer cells while minimizing damage to healthy tissues. They are a key advancement in precision medicine.
Structure of an ADC:
(1) Monoclonal Antibody (mAb): Binds specifically to a target antigen expressed on the surface of cancer cells. Provides targeting specificity.
(2) Cytotoxic Payload (Drug): A potent chemotherapeutic agent (e.g., auristatins, maytansinoids, or calicheamicins). Kills cancer cells once delivered.
(3) Linker: A chemical bridge connecting the antibody and the drug. Stable in circulation but releases the drug inside the target cell.
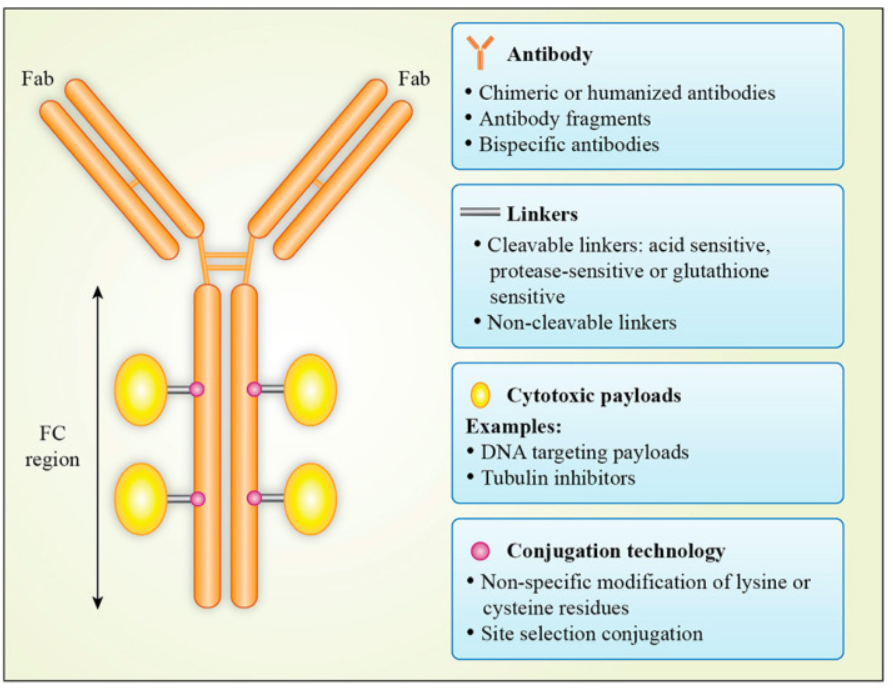
Figure 1:Antibody–drug conjugate structure[1]
Antibody-Drug Conjugate Mechanism
(1) Target Binding
Antibody Specificity: The monoclonal antibody (mAb) component of the ADC is designed to recognize and bind to a specific antigen (protein) that is overexpressed on the surface of cancer cells[2]. Examples of target antigens include HER2 (breast cancer), CD30 (lymphoma), and Trop-2 (triple-negative breast cancer).
Selective Targeting: The antibody ensures that the ADC binds primarily to cancer cells, sparing healthy cells that express little or none of the target antigen.
(2) Internalization
Receptor-Mediated Endocytosis: Once the ADC binds to the target antigen, the ADC-antigen complex is internalized into the cancer cell through a process called endocytosis. The complex is enclosed in a vesicle called an endosome, which then matures into a lysosome.
(3) Linker Cleavage and Drug Release
Lysosomal Degradation: Inside the lysosome, the acidic environment and lysosomal enzymes (e.g., proteases or cathepsins) break the linker, releasing the cytotoxic payload. Some linkers are designed to be cleaved by specific conditions, such as low pH or enzymatic activity.
Release of Cytotoxic Drug: The drug is freed from the antibody and becomes active.
(4) Cytotoxic Effect
Disruption of Cellular Processes: The released cytotoxic drug interferes with critical cellular processes, such as: Microtubule disruption (e.g., auristatins, maytansinoids). DNA damage (e.g., calicheamicins, pyrrolobenzodiazepines). Inhibition of topoisomerase (e.g., SN-38, deruxtecan).
Induction of Apoptosis: The drug's action leads to cell cycle arrest and programmed cell death (apoptosis).
(5) Bystander Effect (Optional)
Diffusion of Drug: Some ADCs are designed with payloads that can diffuse out of the target cell and into neighboring cancer cells, even if they do not express the target antigen. This "bystander effect" enhances the ADC's ability to kill heterogeneous tumors with mixed antigen expression.
Advantages of the ADC Mechanism:
(1) Precision: Targets cancer cells specifically, reducing damage to healthy tissues.
(2) Potency: Delivers highly toxic drugs that would be too harmful if administered systemically.
(3) Versatility: Can be designed to target a wide range of cancer types and antigens.
Challenges in the ADC Mechanism:
(1) Antigen Heterogeneity: Not all cancer cells may express the target antigen.
(2) Resistance: Cancer cells may develop resistance to the ADC or its payload.
(3) Off-Target Toxicity: Premature drug release or binding to healthy cells can cause side effects.
Application of Antibody-Drug Conjugate
Antibody-drug conjugates have revolutionized cancer therapy by combining the precision of monoclonal antibodies with the potent cell-killing ability of cytotoxic drugs. Their applications span a wide range of cancers and are continually expanding as new targets and technologies emerge[3]. Below are the key applications of ADCs in oncology and beyond:
(1) Cancer Therapy
ADCs are primarily used to treat various types of cancers, particularly those that are difficult to treat with conventional therapies. They are especially effective in targeting cancers with specific surface antigens.
(2) Overcoming Drug Resistance
ADCs can deliver highly potent cytotoxic drugs directly to cancer cells, bypassing mechanisms of drug resistance (e.g., multidrug resistance proteins).
For example, Trastuzumab deruxtecan (Enhertu) has shown efficacy in HER2-low breast cancer, expanding the patient population that can benefit from HER2-targeted therapies.
(3) Combination Therapies
ADCs are increasingly being used in combination with other cancer treatments, such as:
Immunotherapy (e.g., checkpoint inhibitors): Enhances the immune response against cancer cells.
Chemotherapy: Synergistic effects with traditional cytotoxic agents.
Radiation therapy: Increases the sensitivity of cancer cells to radiation.
(4) Expanding to New Targets
Researchers are developing ADCs targeting novel antigens expressed on cancer cells, such as: B7-H3 (CD276): Expressed in various solid tumors. PSMA (prostate-specific membrane antigen): For prostate cancer. EGFR (epidermal growth factor receptor): For lung and colorectal cancers.
(5) Beyond Oncology
While ADCs are primarily used in cancer therapy, their potential applications are expanding to other diseases:
Infectious Diseases: ADCs could target pathogens (e.g., bacteria or viruses) by delivering antimicrobial agents directly to infected cells.
Autoimmune Diseases: ADCs could selectively deplete immune cells involved in autoimmune disorders.
Neurological Disorders: ADCs could deliver therapeutic agents across the blood-brain barrier to treat conditions like Alzheimer's or brain tumors.
Prospects of Antibody-Drug Conjugate and FDA-Approved ADC Therapies
Antibody-drug conjugates (ADCs) represent a rapidly evolving field in cancer therapy, with significant prospects for future development and expansion. As of now, several ADCs have been approved by the U.S. Food and Drug Administration (FDA), and many more are in clinical trials.
Prospects of Antibody-Drug Conjugates:
(1) Expanding Target Antigens
Researchers are identifying new tumor-specific antigens to develop ADCs for a broader range of cancers.
(2) Improved Payloads
Development of more potent cytotoxic drugs with novel mechanisms of action (e.g., DNA crosslinkers, RNA polymerase inhibitors). Payloads with bystander effects to kill neighboring cancer cells, even if they do not express the target antigen.
(3) Advanced Linker Technology
Development of stable linkers that prevent premature drug release in circulation, reducing off-target toxicity. Cleavable linkers that release the payload specifically in the tumor microenvironment or inside cancer cells.
(4) Next-Generation ADCs
Dual-targeting ADCs: Simultaneously target two different antigens to improve specificity and reduce resistance.
Non-internalizing ADCs: Release payloads in the tumor microenvironment to target stromal cells and immune cells.
Pro-drug ADCs: Activate the cytotoxic drug only after reaching the tumor site.
(5) Combination Therapies
ADCs are being combined with immunotherapy (e.g., checkpoint inhibitors), chemotherapy, and radiation therapy to enhance efficacy.
Synergistic effects with immune-modulating agents to boost anti-tumor immune responses.
(6) Broader Applications
Beyond oncology, ADCs are being explored for infectious diseases, autoimmune disorders, and neurological conditions.
Potential to deliver therapeutic agents across the blood-brain barrier for brain tumors or neurodegenerative diseases.
(7) Personalized Medicine
ADCs can be tailored to individual patients based on the expression of specific antigens on their cancer cells.
Biomarker-driven approaches to identify patients most likely to benefit from ADC therapies.
(8) Pediatric Cancers
Development of ADCs for pediatric cancers, where targeted therapies are needed to reduce long-term side effects of traditional chemotherapy.
FDA-Approved ADC Therapies:
As of October 2023, the FDA has approved several ADCs for the treatment of various cancers. Below is a list of these therapies:
|
Drug Name |
Target | Payload | Approved Indications |
|
Brentuximab vedotin |
CD30 |
Monomethyl auristatin E (MMAE) |
Hodgkin lymphoma. Anaplastic large cell lymphoma (ALCL) |
|
Ado-trastuzumab emtansine (T-DM1, Kadcyla) |
HER2 |
DM1 (a maytansinoid derivative) |
HER2-positive breast cancer (adjuvant and metastatic settings) |
|
Trastuzumab deruxtecan (Enhertu) |
HER2 |
Deruxtecan (a topoisomerase I inhibitor) |
HER2-positive breast cancer, HER2-low breast cancer, HER2-positive gastric cancer |
| Sacituzumab govitecan (Trodelvy) |
Trop-2 |
SN-38 (a topoisomerase I inhibitor) |
Triple-negative breast cancer (TNBC), Urothelial cancer |
| Gemtuzumab ozogamicin (Mylotarg) | CD33 | Calicheamicin (a DNA-damaging agent) | Acute myeloid leukemia (AML) |
| Polatuzumab vedotin (Polivy) | CD79b | MMAE | Diffuse large B-cell lymphoma (DLBCL) |
| Enfortumab vedotin (Padcev) | Nectin-4 | MMAE | Urothelial cancer |
| Belantamab mafodotin (Blenrep) | BCMA (B-cell maturation antigen) | Monomethyl auristatin F (MMAF) | Relapsed or refractory multiple myeloma |
| Loncastuximab tesirine (Zynlonta) | CD19 | Pyrrolobenzodiazepine (PBD) dimer | Relapsed or refractory diffuse large B-cell lymphoma (DLBCL) |
| Tisotumab vedotin (Tivdak) | Tissue factor (TF) | MMAE | Recurrent or metastatic cervical cancer |
More ADCs are expected to gain FDA approval as clinical trials demonstrate their efficacy and safety. ADCs are being adopted worldwide, with regulatory approvals in Europe, Japan, and other regions.
References
[1] Hafeez U, Parakh S, Gan HK, Scott AM. Antibody-Drug Conjugates for Cancer Therapy. Molecules. 2020 Oct 16;25(20):4764. doi: 10.3390/molecules25204764. PMID: 33081383; PMCID: PMC7587605.
[2] Panowski S , Bhakta S , Raab H ,et al.Site-specific antibody drug conjugates for cancer therapy[J].Mabs, 2014, 6(1):34-45.DOI:10.4161/mabs.27022.
[3] Chari R V J , And M L M , Widdison W C .Antibody–Drug Conjugates: An Emerging Concept in Cancer Therapy[J].Angewandte Chemie International Edition, 2014, 53(15):3796-3827.DOI:10.1002/anie.201307628.