2025-04-15 Hits(112)
Antibody Production
Introduction of Antibodies
Antibody is an Immunoglobulin (Ig) secreted by plasma cells (effector B cells), which plays a crucial role in the immune system. Antibodies specifically recognize and bind to antigens, such as pathogens, toxins, or foreign bodies, thereby neutralizing or labeling them for other immune cells to clear.
The basic structure of the antibody molecule is a Y-shaped molecule formed by two identical heavy chains (H chain) and two identical light chains (L chain) connected by disulfide bonds. Each antibody molecule has two identical antigen-binding sites, located on two Y-shaped arms.
Variable region (V region) : Located at both ends of the antibody, it is responsible for the specific recognition and binding of antigens.
Constant region (C region) : constitutes the backbone of the antibody and determines the effector functions of the antibody, such as activation of the complement and binding of the Fc receptor.
Classification of Antibodies
Depending on the heavy chain constant region, antibodies can be divided into five main types, namely IgA, IgD, IgE, IgG, and IgM, which play different roles in the immune response and have their own unique structural and functional characteristics:
|
Antibody Types |
Structure | Characteristics | Function |
|
IgG |
Consists of two heavy chains (gamma chains) and two light chains. | It is the highest content of antibodies in the serum, accounting for 70-75% of the serum immunoglobulin. | Through the placenta, it provides passive immunity to the newborn. Neutralize viruses and toxins. Activation of complement classical pathway. Involved in antibody-dependent cell-mediated cytotoxicity (ADCC). |
|
IgM |
Consists of two heavy chains (μ chains) and two light chains, usually in pentamer form. | It is the first antibody produced in the initial immune response. | Has a strong agglutination antigen and the ability to activate complement. Due to its pentamer structure, it can effectively neutralize pathogens. |
|
IgA |
Consists of two heavy chains (α chains) and two light chains, there are monomer and dimer forms. |
Mainly found in mucosal surface, secretions (such as saliva, tears, milk) and intestinal tract. |
Provides mucosal immunity to prevent pathogens from invading through the mucosa. The dimer form is connected by J chain to enhance its stability. |
| IgD | Consists of two heavy chains (δ chains) and two light chains. |
The serum content is low, the function is not completely clear. |
May be involved in the initiation and regulation of immune response. As a receptor on the surface of B cells, it is involved in the activation of B cells. |
| IgE | Consists of two heavy chains (ε chains) and two light chains. | The serum content is the lowest, and is closely related to allergic reaction and parasitic infection. | Binds to receptors on the surface of mast cells and basophils to mediate allergic reactions. Participate in the fight against parasitic infections. |
Each antibody type has its specific biological function and mechanism of action, which together constitute an important part of the body's immune defense. These antibodies play unique roles in different types of immune responses and disease states.
In addition, antibodies are also divided into monoclonal antibodies and polyclonal antibodies, which differ in terms of preparation, specificity, and application:
|
Antibody Types |
Preparation Method | Specificity | Application | Advantages | Disadvantages |
| Monoclonal Antibody | Produced from a single B cell clone, usually prepared by hybridoma technology. Hybridomas are formed by the fusion of immune B cells with myeloma cells, capable of multiplying indefinitely and producing antibodies with the same specificity. | Highly specific, recognizing only one epitope. The antibody properties are uniform and the consistency between batches is high. | Used in diagnostic reagents, such as flow cytometry, immunohistochemistry, etc. It is used for treatment, such as cancer immunotherapy and autoimmune disease treatment. For scientific research, such as antigen purification, immunoprecipitation, etc. | Strong specificity, less cross reaction. Can be produced in large quantities, stable in nature. | The preparation process is complex and the cost is high. May cause an immune response, such as an anti-mouse antibody response. |
| Polyclonal Antibody | It is produced from multiple B cell clones and is usually prepared by collecting serum after animal immunization. After immunizing animals, the serum contains a variety of antibodies against different antigen epitopes. | Multi-specificity, recognition of multiple epitopes. The antibody properties are heterogeneous and the consistency between batches is low. | Used in diagnostic reagents, such as ELISA, Western Blot, etc. Used in treatment, such as immunotherapy of certain infections. For scientific research, such as antigen detection, immunoprecipitation, etc. | The preparation is relatively simple and the cost is low. Multiple epitopes can be recognized to increase the sensitivity of detection. | Lower specificity and more cross-reactivity. Poor batch to batch consistency, difficult to standardize. |
In summary, monoclonal antibodies are highly specific and uniform, suitable for applications that require precise identification of specific antigens, but are complex and costly to prepare. Polyclonal antibodies have multispecificity and high sensitivity, and are suitable for applications that detect multiple antigens or require high sensitivity, but have low specificity and poor batch to batch consistency. When choosing whether to use monoclonal antibodies or polyclonal antibodies, it is necessary to decide according to the specific application needs, experimental purposes and cost considerations.
Method of Antibody Production
(1) Hybridoma technology
Hybridoma cells were formed by fusion of antibody-producing B cells with myeloma cells that proliferate in vitro by cell hybridization. The hybridoma cells have both the characteristics of B-cell synthesis of specific antibodies and the characteristics of myeloma cell proliferation in vitro, so that a large number of monoclonal antibodies against specific antigenic domains can be prepared.
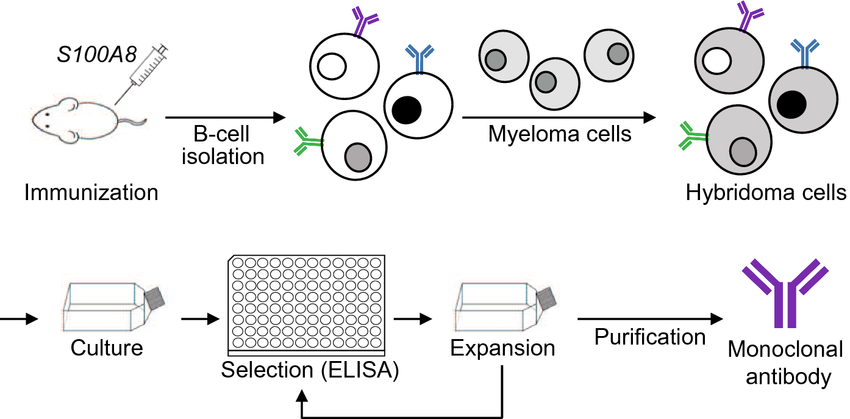
Figure 1:Schematic representation of monoclonal antibody production using hybridoma technology[2]
(2) Phage display technology
Phage display technology is an efficient gene expression screening technology, which fuses foreign proteins or peptides with specific phage capsid proteins, displays them on the phage surface and maintains relatively independent spatial conformation and biological activity, which is conducive to the specific recognition and binding of target molecules, so as to realize the unity of genotype and expression type.
phage display technology is to insert the gene encoding exogenous peptides or proteins into the appropriate position of the structure gene of the phage coat protein through genetic engineering technology, so that the gene can be correctly expressed in the reading frame, so that the exogenous peptides or proteins can form fusion proteins on the capsid protein of the phage and be presented on the surface of the phage with the reassembly of the progeny phage. It can maintain relative spatial structure and biological activity. Then the target molecules were used to wash away the unspecifically bound bacteriophages by appropriate panning methods. Then the bound phage is eluted with acid-base or competing molecules, and the neutralized phage infects E. coli with amplification. After 3-5 rounds of enrichment, the proportion of phages that can specifically recognize target molecules is gradually increased, and finally the polypeptide or protein that can recognize target molecules is obtained.
(3) Single B cell antibody technology
As a new generation of antibody development technology, single B cell antibody technology can efficiently and rapidly isolate antibodies from single B cells, which is another breakthrough in antibody generation technology after hybridoma and phage display. According to the characteristics that each B cell contains only one functional heavy chain variable region DNA sequence and one light chain variable region DNA sequence and produces only one specific antibody, B cells are collected from animal lymphoid tissue, and heavy chain and light chain variable region genes are amplified from single antibody-secreting B cells by monoclonal technology. The collected B cells were then amplified into a single clonal population of origin. Then the host of antibody production is selected and the bioactive monoclonal antibody is expressed in mammalian cells. Single B cell technology has outstanding advantages such as high specificity, high activity and high affinity. At present, antibodies prepared by single B cell technology have been widely used in biomedical research, diagnostic tools and therapeutic drug research and development.
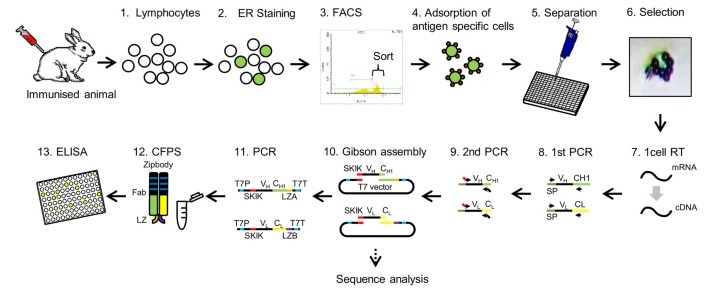
Figure 2:Rapid monoclonal antibody screening of single B cells using free protein synthesis to form antigen-binding fragments[3]
(4) Genetic engineering technology
Genetic engineering technology antibody production is a method of using recombinant DNA technology to prepare antibodies, this method can produce highly specific and pure monoclonal antibodies, suitable for large-scale production, to meet clinical and commercial needs, can be modified through genetic engineering technology, such as humanization, increase effect function. Genetic engineering technology to produce antibodies has important applications in medical diagnosis, treatment and biological research, and is an important part of modern biotechnology. The basic steps of recombinant antibody production by genetic engineering technology include immunizing animals, B cell isolation and RNA extraction, cDNA synthesis, antibody gene cloning, expression vector construction, transfection of host cells, antibody expression and screening, and antibody purification.
Current Status and Prospect of Antibody Therapy
The global antibody drug market has grown rapidly in recent years and is expected to continue to grow in the future. Antibody drugs occupy an important position in the modern biomedicine market, accounting for about 40% of the total biologics market.
Antibody drugs are widely used in the treatment of many diseases such as cancer, autoimmune diseases, metabolic diseases and infectious diseases. Monoclonal antibodies are the mainstream therapeutic antibody drugs, accounting for 95% of therapeutic antibody drugs in 2022.
Antibody drugs are highly specific, can reduce the impact on normal cells, work through a variety of mechanisms such as blocking signaling, promoting the immune response, and generally have low toxic side effects and good tolerance. New forms of antibody, such as bisspecific antibody and antibody conjugated drug (ADC), have been emerging, which has injected new vitality into the antibody drug market.
New targets and therapies are constantly being discovered and developed, for example antibody drugs targeting TNFSF15 have shown good efficacy and safety in the treatment of inflammatory bowel disease. Emerging therapeutic strategies such as antibody drug conjugate (ADCs) and cell therapy have made significant advances in cancer treatment. In the future, with the technological progress, the discovery of emerging targets and policy support, its market prospects are very broad.
References
[1] Schook L B .Monoclonal antibody production techniques and applications[J].biochimie, 1987.DOI:10.1016/0300-9084(88)90169-1.
[2] Kim J P , Yun H , Kim E J ,et al.Generation of a novel monoclonal antibody against inflammatory biomarker S100A8 using hybridoma technology[J].Biotechnology Letters, 2023.DOI:10.1007/s10529-023-03364-0.
[3] Ojima-Kato, T., Nagai, S. & Nakano, H. Ecobody technology: rapid monoclonal antibody screening method from single B cells using cell-free protein synthesis for antigen-binding fragment formation. Sci Rep 7, 13979 (2017). https://doi.org/10.1038/s41598-017-14277-0.