2023-07-20 Hits(525)
Antibody sequencing refers to the sequence determination of antibody proteins from beginning to end; Monoclonal antibody sequencing refers to the process of sequencing antibodies produced by a single B cell.
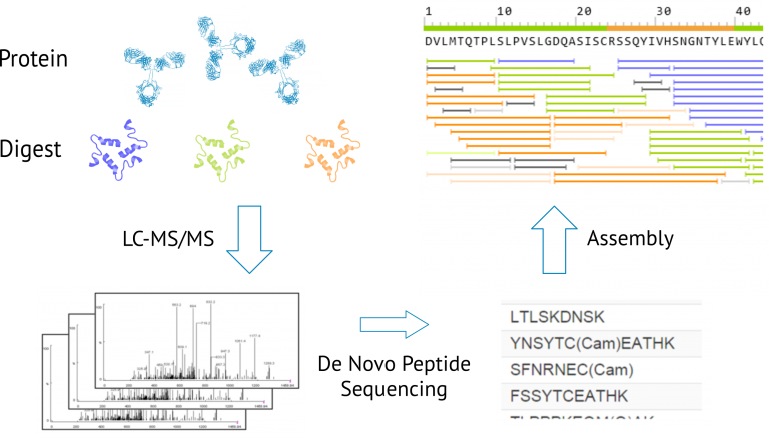
Figure 1: Antibody sequencing
The mass of the amino acid residues of the protein is determined by measuring the mass difference between two fragment ions of the tandem mass spectrometry, and then along the main chain of the protein to determine each amino acid, and finally the complete sequence of the amino acid is obtained.
The gene sequence of monoclonal antibodies can be obtained through antibody sequencing service, and the target antibody can be obtained by recombinant expression to ensure the stability of the antibody and effectively avoid endogenous biological fouling. Antibody sequencing has a wide range of application spaces: (1) antibody drug development: accurate primary structure information is the premise of drug research and development, and the foundation for the research and development of antibody drugs is laid by conducting antibody sequencing analysis; (2) Antibody engineering research: the obtained antibody sequences are recombinant expression, which can express various forms of recombinant antibodies, and can also realize antibody humanization; (3) Patent application: provide monoclonal antibody sequence information for applying for protective patents.
KMD Bioscience has a wealth of experience in antibody sequencing with skilled laboratory technicians who deliver high-quality sequencing results in a short cycle time. We use the high-quality Orbitrap Fusion Lumos mass spectrometer and full-featured antibody sequencing data processing software PEAKS AB Software, combined with gene sequencing and bioinformatics databases, to establish a new generation of antibody sequencing platform, which can achieve rapid and accurate analysis of antibody primary structure, to ensure the accuracy of each antibody sequencing.
Monoclonal antibody is an antibody against a single epitope produced by hybridoma cells, and the gene sequence of monoclonal antibody of hybridoma cells can be obtained through monoclonal antibody sequencing, and its operation process is consistent with hybridoma cell sequencing. The main processes include RNA extraction, cDNA cloning, T vector construction, antibody gene sequencing, bioinformatics analysis, and project report.
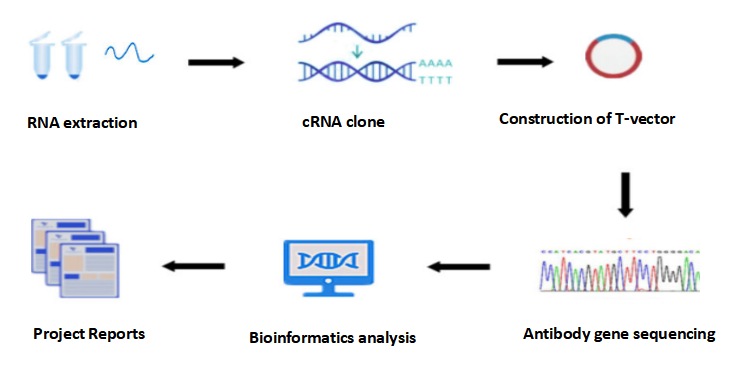
Figure 2: The process of Hybridoma sequencing
5.1.1 Hybridoma Production and total RNA Extraction
Total RNA was extracted from hybridoma cells using TRIzol reagent according to the manufacturer's instructions.
5.1.2 Reverse Transcriptional Synthesis Antibody Variable Region cDNA
A reverse transcriptase kit was used with additional RNA samples (hybridoma RNA samples from mouse antibodies or RNA samples from chimeric antibodies), primers, 10 mM deoxynucleotide triphosphate mixture (dNTPs), H2O, and 80U/ml RNase inhibitor. Note: Due to its RNA content, the template was converted into oligonucleotides and stored at -80°C.
5.1.3 PCR Amplification of the Antibody Variable Region
Set up a PCR reaction for each cDNA synthesis, followed by buck PCR according to thermal cycler conditions, and finally take 5 μl per RT-PCR reaction to run on a 1% agarose gel in 90 V TAE buffer.
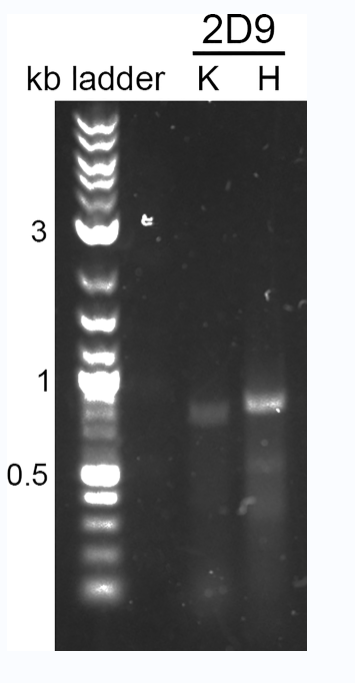
Figure 3: PCR amplification of the antibody variable region
5.1.4 Construction of T-vector
Use the PCR cleaning and gel extraction kit of Macherey-Nagel to clean the RT-PCR reaction. According to the manual of the flat-end cloning kit, 2l of each PCR-washed product was flat-ended cloned into the pCR-Blunt-II-TOPO vector. Next, 3l of each TOPO cloning reaction was transformed into chemically competent E. coli. A total of 100l of each transformation was spread onto LB plates containing 50g/ml kanamycin and incubated overnight at 37°C.
5.1.5 Antibody Gene Sequencing
After colonies were obtained, 5-10 colonies of each antibody chain were inoculated in 5ml LB/ kanamycin medium and shaken overnight at 37°C at 250rpm. These cultures were prepared in small quantities using Macherey-Nagel's small quantity production kit, and the resulting plasmid DNA was Sanger sequenced by Sequetech Corporation using M13 forward primers.
5.1.6 Bioinformatics analysis
Sequencing data were analyzed using a custom Python program available on GitHub to remove non-functional DNA.
5.1.7 Project reports
Delivery the antibody sequences and experimental reports.
5.2 Antibody sequencing
The main process of antibody sequencing is as follows: production of samples to be tested, different digestions, mass spectrometry analysis, sequence verification, data analysis, and delivery the report.
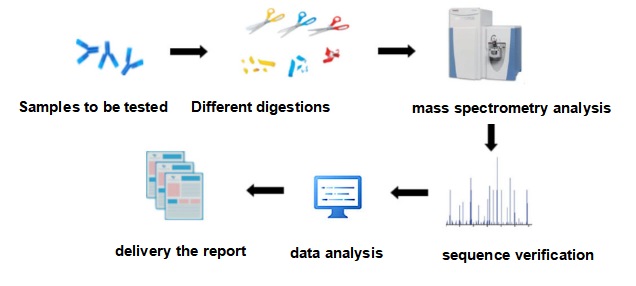
Figure 4: The process of antibody sequencing
5.2.1 Prepare the sample to be tested
Customers need to provide protein samples with a purity greater than 95% 200-300μg.
5.2.2 Different digestions
To ensure full sequence coverage, up to 6 different proteases will be used to digest the target protein for different project needs, and at least 10 fragments of a single amino acid will be generated, and the full sequence splicing between different light and heavy chain digestion peptides will be automatically realized by PEAKs Studio and PEAKs Ab software; Leverage both manual and database for two-step verification.
5.2.3 Mass spectrometry analysis
The laboratory uses the Orbitrap Fusion Lumos mass spectrometer to distinguish different variants of homologous amino acids and accurate determination of leucine and isoleucine can be achieved by the characteristic peaks of the secondary fragmentation fragment. At the same time, the system can also distinguish amino acid molecules for post-transcriptional modification.

Figure 5: Orbitrap Fusion Lumos mass spectrometer
5.2.4 Sequence verification
A database based on the assay sequence was established, and the acquired mass spectrometry data was re-compared and analyzed using the sequence confirmation mode to ensure that the delivered product was correct.
5.2.5 Data analysis
Use bioinformatics analysis software to remove non-functional antibody genes.
5.2.6 Experimental Report
Submit the experimental report and antibody sequencing report to the customer.
Monoclonal antibody molecules play a key role in drug development and diagnostic reagent development. The primary structure of antibody molecules, especially the amino acid sequence of its CDR region, is the core of its biological function, and it is of great significance for accurate and rapid analysis of its complete sequence. Based on high-resolution mass spectrometry and high-fidelity gene cloning technology, KMD Bioscience can provide customers with high-quality antibody sequencing services.