2024-05-31 Hits(194)
Protocol
The Bradford dye-binding assay is a colorimetric method used to measure total protein concentration. Developed by Marion M. Bradford in 1976, It relies on the binding of Coomassie Brilliant Blue dye to proteins, resulting in a color change that is proportional to the protein concentration and can be measured spectrophotometrically. The assay is appreciated for its simplicity, speed, and sensitivity.
The Bradford Protein Assay is widely used in biochemistry and molecular biology for several compelling reasons:
Quantification of Protein Concentration
The Bradford Protein Assay's primary purpose is to measure the concentration of proteins in a sample accurately. This is essential in various experimental workflows where knowing the exact amount of protein is critical, such as in enzyme kinetics, protein purification, and other biochemical analyses.
Sensitivity and Specificity
Sensitivity: The Bradford assay is highly sensitive, capable of detecting protein concentrations as low as 1 µg/mL. This makes it suitable for analyzing small amounts of protein where other methods might not be effective.
Specificity: The Coomassie Brilliant Blue dye used in the assay specifically binds to proteins, particularly at arginine, lysine, and histidine residues, which enhances its specificity compared to other colorimetric methods.
Speed and Simplicity
Rapid Procedure: The Bradford assay can be completed in a matter of minutes, making it ideal for high-throughput settings and routine protein analysis.
Simple Protocol: The assay involves minimal sample preparation and straightforward pipetting steps, making it accessible for researchers at all levels of expertise.
Versatility
Wide Range of Applications: The Bradford assay is applicable in various fields including cell biology, microbiology, and immunology. It is used to quantify proteins in cell lysates, culture media, purified protein preparations, and more.
Compatibility with Various Sample Types: The assay can be used with a wide range of sample types, including whole cells, tissue extracts, and purified proteins.
Non-Destructive Method
The Bradford assay is non-destructive, meaning that it does not alter the protein sample significantly. This allows the same sample to be used for other analyses if needed.
Cost-Effective
The reagents and materials required for the Bradford assay are relatively inexpensive, making it a cost-effective option for routine protein quantification.
High Throughput Capability
The assay is easily adaptable to 96-well plate formats, allowing for high-throughput screening of multiple samples simultaneously. This is particularly useful in research settings where large numbers of samples need to be analyzed.
Reagent: Dilute 5x Bradford Reagent (BioRad) in a 4:1 ratio with water, then filter using gravity filtration.
Equipment: Use a 96-well ELISA plate (Limbro microtitration plate) and the Spectrophotometer or plate reader capable of reading at 595 nm (BioRad model 2550 EIA reader).

Figure 1 Source: Wikipedia
Preparation:
Dilute the 5x Bradford Reagent in a 4:1 ratio with distilled water and filter the solution using a gravity filter.
Turn on the spectrophotometer or plate reader to allow the bulb to warm up for approximately 10 minutes before use.
Standard Preparation:
Prepare a set of protein standards with known concentrations using a stock protein solution. For BSA, typical standards range from 0-1 mg/mL.
Example dilutions for a 1 mg/mL BSA stock solution:
0.0 mg/mL: 0 µL BSA + 30 µL buffer
0.2 mg/mL: 6 µL BSA + 24 µL buffer
0.4 mg/mL: 12 µL BSA + 18 µL buffer
0.6 mg/mL: 18 µL BSA + 12 µL buffer
0.8 mg/mL: 24 µL BSA + 6 µL buffer
1.0 mg/mL: 30 µL BSA + 0 µL buffer
Plating Standards and Samples:
Plate the standards in triplicate by adding 10 µL of each standard solution to the wells, starting from column 2 (leave column 1 blank for background correction).
Plate your samples in triplicate, ensuring their concentrations fall within the range of the standard curve (0-1 mg/mL for BSA).
Adding Bradford Reagent:
Add 200 µL of the diluted Bradford reagent to each well-containing standards and samples.
Allow the mixture to stand for 5 minutes to ensure complete binding and color development.
Measurement:
Insert the 595 nm filter into the spectrophotometer or plate reader.
Place the ELISA plate on the reader, press "blank" to calibrate using the blank wells, and then press "start" to measure the absorbance of the samples and standards.
Record the absorbance values once the reading is complete.
Data Analysis:
Plot the absorbance values (y-axis) against the known protein concentrations (x-axis) to create a standard curve.
Use the standard curve to determine the concentration of unknown protein samples by interpolating their absorbance values.
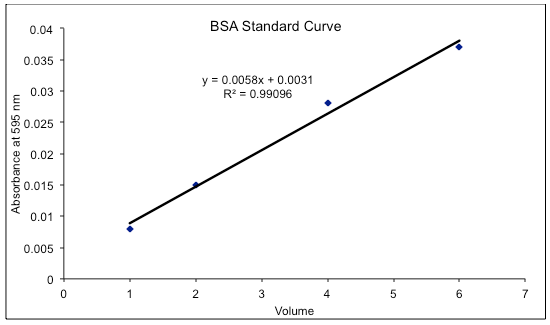
Figure 2 Source: Wikipedia
Note: BSA is not the most accurate standard for the Bradford assay. Multiply the results by 2.1 for a closer approximation of protein concentration. Lysozyme, ovalbumin, and catalase are better standards for this assay and do not require this adjustment.
Tips: Detergents and Alkali: Avoid using detergents and strongly alkaline solutions as they interfere with the Bradford assay.
Warming Up: Ensure the EIA reader is adequately warmed up before use to ensure accurate readings.
Standard Curve: Always prepare a fresh standard curve for each assay to ensure accuracy.