2024-11-06 Hits(113)
Protein Expression
In 1993, a natural antibody lacking a light chain (VL) was discovered in the serum of camelids. Its structure is simple, consisting only of two heavy chains (VH), and its volume is very small, about 15 kDa, hence it is called nanobody or single domain antibody. Later, this antibody was also found in animals such as alpacas and sharks. Based on its unique structure and broad application prospects, the preparation of nanobodies has a broad application market, especially in the field of drug development. Due to the strong penetration of nanobodies, it has become an ideal choice for treating tumor diseases, which helps to improve the efficacy of drugs and reduce side effects. Although antibodies with similar structures also exist in shark animals, most single domain antibody development is carried out through alpaca immunization due to the ease of breeding and immunization, as well as the high profitability of animals such as alpacas.
The immunogens used in alpaca immunization mainly include natural antigens, recombinant antigens, synthetic antigens, and small molecule antigens. The purification of natural antigens is difficult and costly. Although recombinant antigens differ in conformation from natural antigens, they can be produced in large quantities. The preparation of synthetic antigens can be achieved through in vitro synthesis methods, mainly small molecule protein or peptide antigens, whose structures are controllable but may be complex in design. Small molecule antigens are mostly small molecule compounds such as oligosaccharides and nucleotides. Due to their lack of immunogenicity, they can only be used as immunogens after being attached to large molecule carriers.
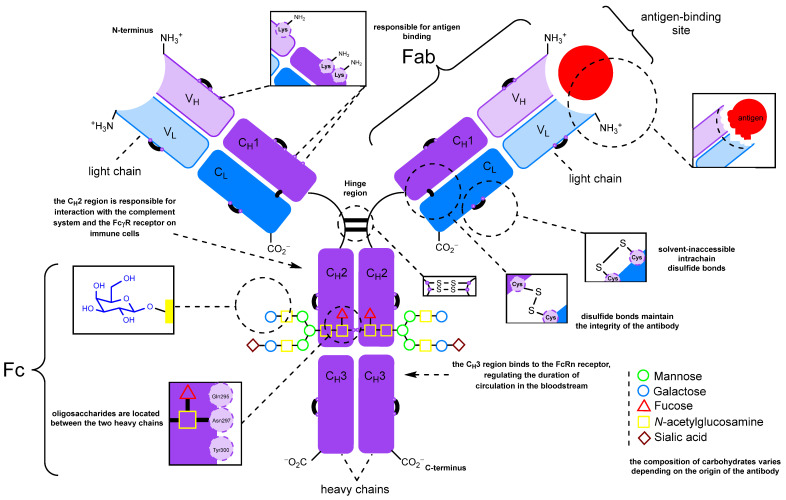
Fig. 1 The structure and modifiable sites of immunoglobulin G
(Figure Source:Introduction of Carbonyl Groups into Antibodies - PMC (nih.gov))
Preparation of synthetic antigens
When preparing protein immunogens, the difference between the target protein and the host system can lead to differences in protein expression levels and stability, making it difficult to prepare high expression and high purity proteins. The selection of an appropriate protein expression system is crucial as some proteins require specific post-translational protein modifications to maintain their antigenicity. In order to maintain the natural conformation of proteins and stimulate corresponding immune responses in the body, it is necessary to add cofactors and special conditions. When preparing small molecule immunogens, they need to be conjugated with carrier proteins to enhance immunogenicity. However, the efficiency of conjugation may be low and the process may be complex, and the stability of the conjugated immunogens during transportation is difficult to maintain. When preparing peptide immunogens, if the desired peptide is a long-chain peptide, the preparation process is relatively complex, especially for peptides with complex sequences. The difficulty of peptide synthesis and purification is high, and not all peptide sequences have sufficient immunogenicity. In order to have correct epitope display, special carriers need to be designed so that peptides can display antigen epitopes in appropriate conformations.
For specific expression systems, the codons of target genes are optimized and strong promoters are designed to increase protein expression levels. The use of adjuvants can enhance the immune system's response to immunogens during the immune process, and multiple immunizations can gradually increase the immune system's response.
When conducting subsequent peptide and small molecule screening, it is important to ensure that specific antibodies targeting the target peptide or small molecule can be identified and isolated during the screening process, avoiding interference from non-specific antibodies. During the screening process, it is also necessary to maintain the stability of peptides and small molecules to avoid their degradation or denaturation. It is best to choose a high-sensitivity screening method when conducting screening, so that low concentrations of antibodies or antigens can also be detected.
Key points of immunogen preparation
When preparing protein immunogens, different protein expression systems should be selected based on the characteristics of the target protein, such as E. coli, yeast, insect cells, or mammalian cells. By adjusting the temperature and optimizing the induction time, the stability and solubility of proteins can be improved. The use of appropriate salts, pH buffering agents, and stabilizers such as glycerol during the purification process can maintain the natural conformation of the protein. Introducing specific functional groups onto small molecules through chemical synthesis can enhance the immunogenicity of small molecule immunogens. Common carrier proteins used in small molecule design include KLH and BSA, which can carry multiple small molecule epitopes and enhance the immunogenicity of small molecules. Biocompatible crosslinking agents can be used to achieve stable coupling between peptides and carrier proteins, ensuring the stability and activity of peptide synthesis immunogens in vivo. The appropriate peptide sequence length is selected for peptide sequence design, typically between 15-20 bases, to ensure the inclusion of sufficient antigenic determinants. Adjuvants can be used during the immune process to enhance the immune system's response to immunogens.
KMD Bioscience has been dedicated to the construction and screening of nanobody libraries for many years, with rich experience in single domain antibody production. Based on our mature antibody discovery service platform, hundreds of alpaca nanoantibody library construction services are successfully delivered every year. KMD Bioscience has established a comprehensive and mature nanobody preparation service platform. Based on phage display technology, we can provide main experimental steps including antigen design, alpaca immunization, alpaca nanoantibody library construction and screening, and activity function verification, and provide customers with high specificity and affinity alpaca nanoantibody library construction services. And we will conduct comprehensive analysis of the nano sequence information and verify it through various experiments, such as EC50 determination, affinity analysis, flow cytometry verification, etc. We have multiple phage antibody library construction platforms including M13, T4, T7, and λ phages, which can meet the different needs of customers and provide personalized single domain antibody development services. KMD Bioscience specializes in building different types of phage display libraries, such as immune libraries, natural libraries, semi synthetic libraries, synthetic libraries, etc. The nanobody library we have constructed has a large capacity and can produce high affinity nano antibodies. We can provide customers with various bacteriophage vectors including pMECS, pComb3X, and pCANTAB 5E. We have strains such as TG1 Escherichia coli, XL1 Blue, and ER2738, which can be used for phage infection after expanded cultivation. The antibody library we have built has a large capacity of up to 10^9, with a high insertion rate of target fragments, which is beneficial for screening nanobodies that satisfy customers. We can also express and purify the selected nanobodies according to customer needs. In addition to prokaryotic expression systems, we also have various eukaryotic expression systems for antibody proteins, such as mammalian cells, yeast cells, plant and insect cell expression systems, etc., which can produce high-quality nanobodies for customers.
Reference
[1] Sethu S, Govindappa K, Alhaidari M, et al. 2012 Oct;60(5):331-44. doi: 10.1007/s00005-012-0189-7. Epub 2012 Aug 29. PMID: 22930363.
[2] De Groot AS, Khan S, Mattei AE, et al. Does human homology reduce the potential immunogenicity of non-antibody scaffolds? Front Immunol. 2023;14:1215939.
[3] Kozma GT, Shimizu T, Ishida T, et al. Anti-PEG antibodies: Properties, formation, testing and role in adverse immune reactions to PEGylated nano-biopharmaceuticals. Adv Drug Deliv Rev. 2020;154-155:163-175.