2025-04-09 Hits(75)
Antibody Production
Introduction of Polyclonal Antibodies
Polyclonal antibodies are a mixture of antibodies produced by different B cell clones in the body, each recognizing a distinct epitope (specific binding site) on the same antigen. They are generated in response to an antigen, typically through immunization of an animal (e.g., rabbit, goat, or mouse), and are harvested from the serum of the immunized host.
Polyclonal antibodies are a diverse mixture of antibodies that bind to multiple epitopes on the same antigen. They are produced by many different B cell clones, each secreting a unique antibody. Due to their ability to bind multiple epitopes, polyclonal antibodies often have high avidity (overall binding strength) for the target antigen. They can recognize and bind to various regions of an antigen, making them useful for detecting antigens that may have slight variations or denatured forms.
Polyclonal antibodies are used in techniques like ELISA, Western blotting, and immunohistochemistry for detecting and quantifying antigens. Employed in diagnostic assays to identify pathogens or biomarkers. Occasionally used in treatments, such as antivenoms or immunotherapies.
Polyclonal antibodies have several advantages. High sensitivity due to binding to multiple epitopes. Tolerant to minor changes in the antigen's structure. Generally easier and less expensive to produce than monoclonal antibodies. However, it also has many disadvantages. Batch-to-batch variability due to the natural immune response. Potential for cross-reactivity with similar antigens. Less specific than monoclonal antibodies, which target a single epitope. In contrast, monoclonal antibodies are derived from a single B cell clone and bind to a single epitope, offering higher specificity but less versatility in some applications.
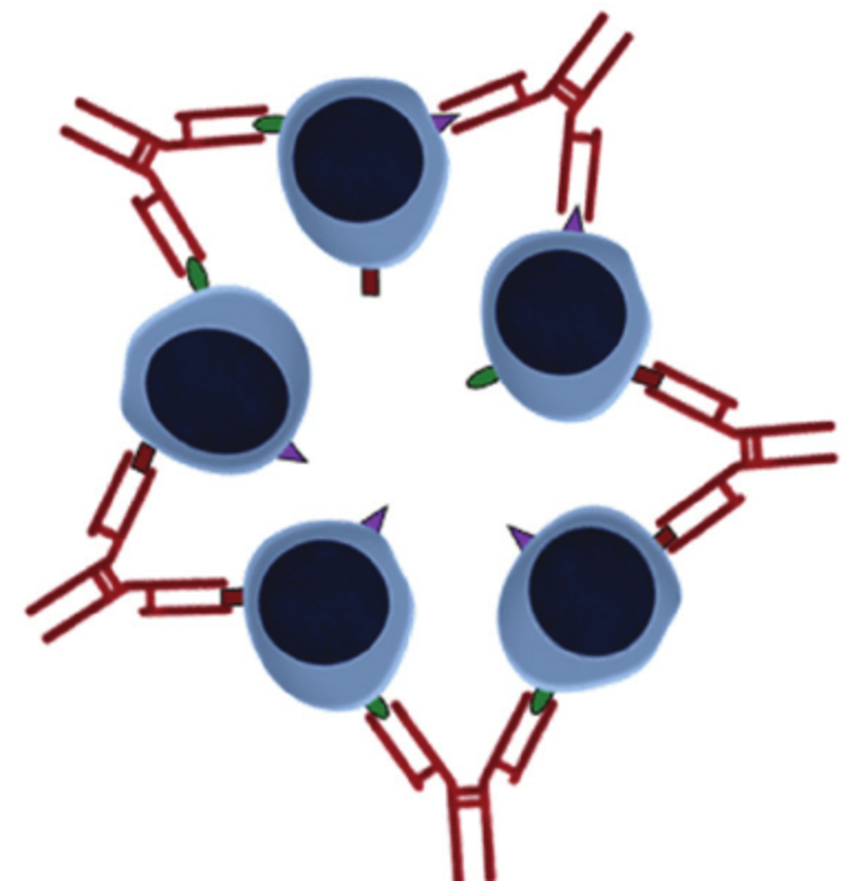
Figure 1:Polyclonal antibody schematic[1]
How to Produce Polyclonal Antibodies?
Polyclonal antibody production involves immunizing an animal with a specific antigen, stimulating its immune system to produce a diverse mixture of antibodies against multiple epitopes on the antigen[2].
Steps to Produce Polyclonal Antibodies:
(1) Antigen Preparation:
Isolate and purify the antigen of interest (e.g., a protein, peptide, or other molecule). Ensure the antigen is immunogenic (capable of eliciting an immune response). If the antigen is small or weakly immunogenic, conjugate it to a carrier protein (e.g., keyhole limpet hemocyanin (KLH) or bovine serum albumin (BSA)) to enhance immunogenicity.
(2) Animal Selection:
Choose an appropriate host animal based on the required antibody volume, specificity, and intended use. Common animals include rabbits, goats, sheep, mice, or chickens. Ensure the animal is healthy and free from infections.
(3) Immunization:
Inject the antigen into the animal, typically mixed with an adjuvant (e.g., Freund’s adjuvant) to enhance the immune response. Administer the antigen via subcutaneous, intramuscular, or intraperitoneal routes. Perform booster immunizations at regular intervals (e.g., 2–4 weeks) to strengthen the immune response and increase antibody production.
(4) Serum Collection:
After the immune response has peaked (usually 4–8 weeks after the initial immunization), collect blood from the animal. Allow the blood to clot, then centrifuge it to separate the serum, which contains the polyclonal antibodies.
(5) Polyclonal antibody Purification:
Purify the antibodies from the serum using techniques such as:
Protein A/G affinity chromatography: Isolates IgG antibodies.
Antigen-specific affinity purification: Uses the antigen to capture specific antibodies.
Remove contaminants and concentrate the antibody solution.
(6) Characterization:
Test the purified antibodies for specificity, affinity, and concentration using methods like ELISA, Western blotting, or immunoprecipitation. Ensure the antibodies recognize the target antigen and do not cross-react with unrelated molecules.
(7) Storage:
Store the purified polyclonal antibodies at -20°C or -80°C for long-term preservation. Add stabilizers (e.g., glycerol) to prevent freezing damage if needed.
Polyclonal vs Monoclonal Antibody
Polyclonal and monoclonal antibodies are both essential tools in research, diagnostics, and therapeutics, but they differ significantly in their production, specificity, and applications.
(1) Definition
Polyclonal Antibodies: A heterogeneous mixture of antibodies produced by different B cell clones[3]. Recognize multiple epitopes on the same antigen.
Monoclonal Antibodies: A homogeneous population of antibodies produced by a single B cell clone. Recognize a single, specific epitope on an antigen.
(2) Production
Polyclonal Antibodies: Produced by immunizing an animal (e.g., rabbit, goat) with an antigen. Multiple B cell clones are activated, leading to a diverse antibody mixture. Antibodies are harvested from the animal’s serum.
Monoclonal Antibodies: Produced using hybridoma technology. Immunize an animal (e.g., mouse) with an antigen. Fuse antibody-producing B cells with immortal myeloma cells to create hybridomas. Screen and select hybridomas that produce the desired antibody. Culture the selected hybridoma to produce identical antibodies.
(3) Specificity
Polyclonal Antibodies: Recognize multiple epitopes on the same antigen. Less specific due to the diversity of antibodies in the mixture. May cross-react with similar antigens.
Monoclonal Antibodies: Recognize a single epitope on the antigen. Highly specific and less likely to cross-react with other molecules.
(4) Affinity and Avidity
Polyclonal Antibodies: High avidity (overall binding strength) due to binding to multiple epitopes. May have varying affinities (binding strength to individual epitopes).
Monoclonal Antibodies: Consistent affinity for a single epitope. Lower avidity compared to polyclonal antibodies (bind to only one epitope).
(5) Applications
Polyclonal Antibodies: Ideal for detecting denatured or partially degraded antigens. Used in techniques like ELISA, Western blotting, and immunohistochemistry. Suitable for detecting antigens with slight variations.
Monoclonal Antibodies: Ideal for applications requiring high specificity, such as diagnostic assays (e.g., pregnancy tests, cancer biomarker detection). Used in targeted therapies (e.g., cancer treatment, autoimmune diseases). Commonly used in flow cytometry and immunoprecipitation.
(6) Advantages
Polyclonal Antibodies:High sensitivity due to binding to multiple epitopes. Tolerant to minor changes in the antigen’s structure. Easier and less expensive to produce.
Monoclonal Antibodies: High specificity and reproducibility. Consistent performance across batches. Suitable for therapeutic use due to precise targeting.
(7) Disadvantages
Polyclonal Antibodies: Batch-to-batch variability due to the natural immune response. Potential for cross-reactivity with similar antigens. Limited supply (dependent on the immunized animal).
Monoclonal Antibodies: More expensive and time-consuming to produce. Less effective for detecting antigens with slight variations. May lose effectiveness if the target epitope is altered.
Application of Polyclonal Antibodies
Polyclonal antibodies are widely used in various fields due to their ability to recognize multiple epitopes on an antigen, making them versatile and highly sensitive tools. Here are some key applications:
(1) Research Applications
① Western Blotting: Detect and quantify specific proteins in complex mixtures. Polyclonal antibodies are often preferred for detecting denatured or partially degraded proteins.
② Enzyme-Linked Immunosorbent Assay (ELISA): Used for quantifying antigens or antibodies in samples. Polyclonal antibodies are effective in capturing or detecting antigens due to their high avidity.
③ Immunohistochemistry (IHC): Localize specific proteins or antigens in tissue sections. Polyclonal antibodies are useful for detecting antigens that may be present in low amounts or have slight variations.
④ Immunofluorescence (IF): Visualize target antigens in cells or tissues using fluorescent labels. Polyclonal antibodies provide strong signals due to their ability to bind multiple epitopes.
⑤ Immunoprecipitation (IP): Isolate and study specific proteins or protein complexes from a mixture. Polyclonal antibodies are effective for pulling down target proteins.
(2) Diagnostic Applications
① Infectious Disease Detection: Identify pathogens (e.g., viruses, bacteria, parasites) in patient samples. Used in diagnostic kits for diseases like HIV, hepatitis, and Lyme disease.
② Autoimmune Disease Testing: Detect autoantibodies in conditions like lupus, rheumatoid arthritis, and celiac disease.
③ Cancer Biomarker Detection: Identify tumor-specific antigens or biomarkers in patient samples.
④ Allergy Testing: Detect allergen-specific antibodies in patient serum.
(3) Therapeutic Applications
① Antivenoms: Polyclonal antibodies are used to neutralize toxins in snake, spider, or scorpion bites. Produced by immunizing animals with venom and harvesting the antibodies.
② Passive Immunization: Provide immediate immunity against pathogens (e.g., rabies, tetanus) by administering antibody-rich serum.
③ Immunotherapy: Used in some treatments to target pathogens or toxins (e.g., botulism antitoxin).
Polyclonal Antibodies FAQs
(1) Why are polyclonal antibodies more sensitive than monoclonal antibodies?
Polyclonal antibodies bind to multiple epitopes on an antigen, increasing the overall binding strength (avidity) and making them more sensitive for detecting low-abundance targets.
(2) Can polyclonal antibodies be used for therapeutic purposes?
Yes, polyclonal antibodies are used in therapies such as: Antivenoms for snake or spider bites. Passive immunization against diseases like rabies or tetanus. Immunotherapy for certain infections or toxins.
(3) What animals are used to produce polyclonal antibodies?
Common animals include: Rabbits (most common for research). Goats, sheep, or horses (for larger antibody quantities). Chickens (for egg-derived antibodies). Mice or rats (for smaller-scale production).
(4) Are polyclonal antibodies specific?
Polyclonal antibodies are less specific than monoclonal antibodies because they recognize multiple epitopes. This can lead to cross-reactivity with similar antigens.
(5) How are polyclonal antibodies stored?
Store at -20°C or -80°C for long-term preservation.
(6) Can polyclonal antibodies be used in Western blotting?
Yes, polyclonal antibodies are commonly used in Western blotting because they can detect denatured or partially degraded proteins, making them highly effective for this technique.
(7) Why do polyclonal antibodies show batch-to-batch variability?
Because they are produced by the natural immune response of an animal, which can vary depending on the animal’s health, age, and immune status. Each batch is a unique mixture of antibodies.
(8) Can polyclonal antibodies be used in flow cytometry?
Yes, but monoclonal antibodies are often preferred for flow cytometry due to their higher specificity. Polyclonal antibodies may be used if the target antigen is highly variable or requires broad detection.
References
[1] Laftavi MR, Sharma R, Feng L, Said M, Pankewycz O. Induction therapy in renal transplant recipients: a review. Immunol Invest. 2014;43(8):790-806. doi: 10.3109/08820139.2014.914326. PMID: 25296234.
[2] Akerström B, Brodin T , Reis K ,et al.Protein G: a powerful tool for binding and detection of monoclonal and polyclonal antibodies.[J].Journal of Immunology, 1985, 135(4):2589.DOI:http://dx.doi.org/.
[3] Hirata Y .Characterization of IL-6 receptor expression by monoclonal and polyclonal antibodies.[J].Journal of Immunology, 1989, 143.DOI:10.1111/j.1574-6968.1977.tb00621.x.