2024-10-15 Hits(126)
Protein Expression
Antibodies are immunoglobulins produced by B lymphocytes that can participate in humoral immunity. It consists of variable domains, light chain variable regions (VL), and heavy chain variable regions (VH), and recombinant antibodies recognize target antigens through its variable fragment. A type of antibody lacking a light chain has been found in the serum of camels, alpacas, and other camelid animals. Its structure is simple and it still has antibody activity without Fc. In the 1980s, BIRD et al. designed single chain antibodies (scFv) as a genetic fusion of VL and VH, which were unlinked by short flexible peptides. ScFv is the smallest binding unit with antibody activity, also known as nanobody. Due to its lack of Fc domain, its volume is very small, about 25 kDa. Compared with full-length antibodies, it is easier to penetrate into tumor tissues and can be applied in targeted cancer therapy drugs. At the same time, it also plays an important role in detection reagents.
Recombinant protein yeast expression system
The structure of recombinant antibodies is complex and most require post-translational modifications, making them suitable for expression in eukaryotic expression systems. However, the cost of large-scale antibody preparation is relatively high. Single chain antibodies have a small volume, simple structure, and can be expressed in various recombinant single chain antibody expression systems without the need for post-translational modifications, making them suitable for large-scale production. The commonly used recombinant antibody expression systems include Escherichia coli expression system, mammalian cell expression system, insect cell expression system, and yeast expression system. Among them, the yeast expression system can perform relatively complete eukaryotic expression and is relatively simple to operate. Using Pichia pastoris for expression does not accumulate ethanol and can achieve the same degree of glycosylation as mammalian cells. When using yeast expression systems, plasmids should be linearized and integrated into the genome to ensure the stable existence of exogenous genes. According to the purpose of gene expression, yeast expression vectors are selected. Yeast expression system vectors can be divided into yeast intracellular expression vectors and yeast extracellular expression vectors. Among them, yeast intracellular expression vectors include DGAP series, pPIC3, 5K. Yeast intracellular expression vectors can express the target protein inside the cell, avoiding excessive glycosylation of yeast and suitable for expressing non glycosylated proteins in the cytoplasm. Yeast intracellular expression vectors have the characteristic of high expression levels, but the subsequent antibody purification process is more complex. The commonly used yeast extracellular expression vector is pPIC9K, which can secrete the target protein into the extracellular space, and the subsequent antibody purification process is relatively simple.
Pichia pastoris and brewing yeast are widely used hosts in yeast expression systems, with advantages similar to mammalian protein expression systems, as well as the ease of operation of prokaryotic cells. At the same time, they have lower production costs and higher productivity. The growth rate of Pichia pastoris is very fast, it can be cultured at high density, and it can achieve intracellular and extracellular expression. As Pichia pastoris can be cultured in protein free media, it is easy to isolate and purify it later. However, the expressed protein may produce mannose residues during post-translational modification, leading to a shorter half-life of the protein and even immunogenicity. Although the yeast expression system has some shortcomings, there are still many single chain antibodies prepared by the yeast recombinant antibody expression system. The antibodies obtained from the yeast recombinant antibody expression system can be used for the development of therapeutic drugs. For example, the single chain antibody Nb11-59 produced in large scale in Pichia pastoris can be used for the treatment of novel coronavirus.
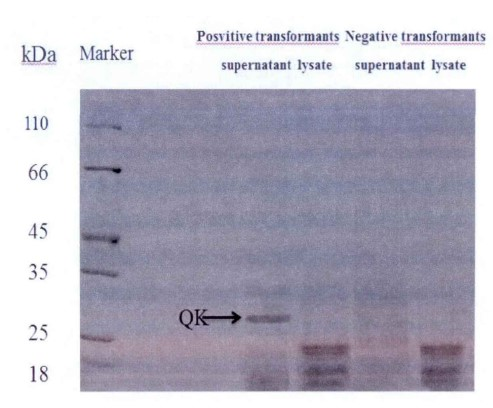
Fig. 1 Identification results of recombinant protein expression in Pichia pastoris GS115
Design of single chain antibodies
When designing single chain antibodies, VH and VL should be selected based on the specificity of the target antigen, and then appropriate linker peptides should be selected to maintain the correct structure of the two. The DNA sequences of VH, VL, and linker are spliced together through gene synthesis to form a complete scFv gene. The main recombinant antibody expression systems for scFv include prokaryotic expression systems and eukaryotic expression systems. Different expression vectors, fusion tags, and host bacteria will produce scFv with different activities. The commonly used expression vectors for preparing single chain antibodies are PET series with strong promoters and terminators, and phage vectors can also be used as an option, such as pIT2. By adding His tags for affinity purification, adding GST tags can increase protein solubility, but in subsequent applications, the tags need to be removed. The intracellular localization of scFv can be observed by adding GFP. When customizing antibodies through yeast expression systems, commonly used yeast host cells include brewing yeast and Pichia pastoris. Among them, brewing yeast has higher safety but lower protein expression levels, which may cause excessive glycosylation and plasmid loss. It is suitable for some proteins that do not require high glycosylation. Pichia pastoris has strong post-translational modification ability, is easy to control expression levels, does not produce ethanol, and can produce a large amount of recombinant proteins. Therefore, Pichia pastoris is an ideal choice for the expression of recombinant single chain antibodies and can be widely used for the preparation of large-scale single chain antibodies.
KMD Bioscience has rich experience in recombinant protein yeast expression services and can provide customers with protein fermentation services of various scales. With our comprehensive protein purification platform, we can provide customers with high-quality recombinant protein products in a short period of time. KMD Bioscience has a complete and mature yeast expression system, providing one-stop services from gene synthesis to yeast protein expression and antibody purification. We have various expression vectors such as pPICZaA, pGAPZaA, pPIC9K, as well as strains such as X33, GS115, and brewing yeast. With large-scale fermentation, we can provide industrial grade services and are equipped with multiple protein expression and purification methods to meet customers' different protein expression needs. We can customize complete protein expression and purification solutions for customers.
Reference
[1] Das PK, Sahoo A, Veeranki VD. Recombinant monoclonal antibody production in yeasts: Challenges and considerations. Int J Biol Macromol. 2024;266(Pt 2):131379.
[2] Boder ET, Wittrup KD. Yeast surface display for screening combinatorial polypeptide libraries. Nat Biotechnol. 1997;15(6):553-7.
[3] Liu CP, Tsai TI, Cheng T, et al. Glycoengineering of antibody (Herceptin) through yeast expression and in vitro enzymatic glycosylation. Proc Natl Acad Sci U S A. 2018;115(4):720-725.