2025-03-31 Hits(76)
Product Target
Introduction to TTP
Thrombotic thrombocytopenic purpura (TTP) is an acute blood disorder characterized by microthrombus formation, platelet reduction, mechanical hemolysis, and organ damage. Delayed treatment of TTP has a high mortality rate, so early diagnosis is critical.
TTP can be divided into (1) rare hereditary congenital TTP (cTTP) and TTP caused by ADAMTS13 gene mutation; (2) Acquired autoimmune TTP (iTTP), caused by its defect ADAMTS13.
Under physiological conditions, ADAMTS13 von Willeilia factor (VWF) molecules cleave to form VWF monomers when released from the endothelial cells of blood vessels. Unlike normal, in TTP, the lack of the ADAMTS13 enzyme causes the unmolested ultra-long VWF molecules to aggregate platelets, thereby forming a microthrombus, causing thrombocytopenia and mechanical fragmentation of red blood cells.
VWF Protein
VWF is a plasma glycoprotein produced by vascular endothelial cells and megakaryocytes and plays an important role in platelet aggregation and coagulation. The VWF gene located on chromosome 12, is approximately 180kb long and contains 52 exons and 51 introns. VWF gene is mainly expressed in vascular endothelial cells and megakaryocytes.
VWF protein consists of the A1 domain and A2 domain. A1 domain binds to platelet membrane glycoprotein Ibα (GPIbα) and participates in the platelet adhesion process. The A2 domain contains the ADAMTS13 cleavage site. VWF binds to ADAMTS13 to split specific peptide bonds in the A2 domain, splitting the super-large VWF polymer into smaller VWF polymers, and avoiding the super-large polymers (>20,000 kDa). Due to the lack of ADAMTS13 in PPT patients, the VWF protein cannot be clipped, causing VWF to recruit too many platelets in the blood and form aggregates, leading to the development of microvascular thrombosis.
VWF protein and mRNA levels vary regionally across the vascular tree, suggesting that the VWF gene is regulated in the systemic circulation.
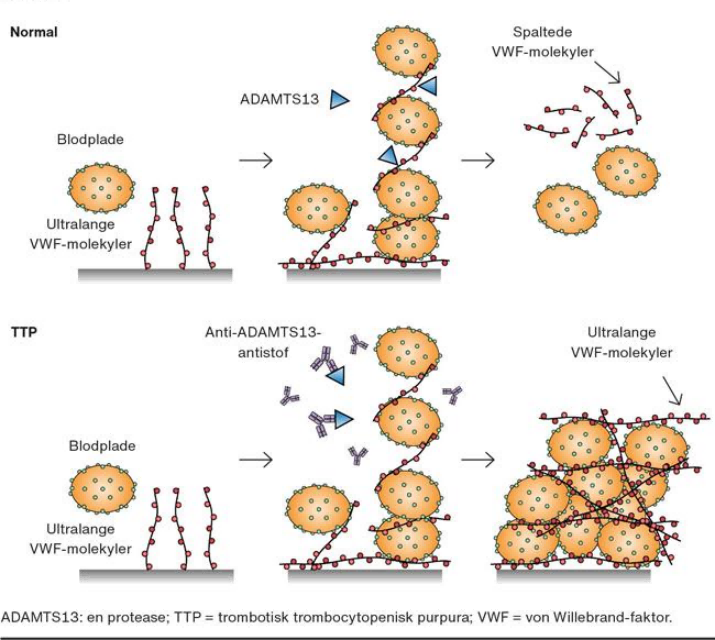
Fig.1 Pathogenesis of TTP. (Reference source: Trombotisk trombocytopenisk purpura)
Clinical Manifestations of VWF
The classic clinical symptoms of VWF are anemia and thrombocytopenia, but some patients experience neurological symptoms such as headache, transient cerebral ischemia, stroke, and loss of consciousness. A small number of patients experience fever, nausea, abdominal pain, and mildly elevated creatinine levels.
ITTP and hTTP cannot be distinguished according to clinical manifestations, but ADAMTS13 conformation (conformational index >0.5) can be used as a basis to judge ITTP.
VWF western blot is an effective method. Tektronix has years of experience in western blot experiments to save you time.
VWF Antibody
The global mortality rate of TTP is still high, and VWF has great prospects for the TTP. The therapeutic targets of TTP include ADAMTS13, anti-AdamTS13 autoantibodies, and VWF, among which the VWF target has attracted much attention.
Targeting VWF as a therapeutic strategy is a hot research topic, and blocks the formation of microthrombus by VWF from binding to platelets.
Anti-VWF aptamer - Caplacizumab is a nano-antibody that prevents the formation of microthrombus by blocking the binding of VWF's A1 domain to platelet GPIbα. Caplacizumab has a high safety profile and short-term efficacy.
Acetylcysteine is a reductase that changes the size of VWF polymers by reducing disulfide bonds between polypeptide chains, resulting in reduced ability to adhere to platelets and is suitable for plasma replacement.
VWF Antibody Case
It has been reported that natural sarcoglycan and hydrolyzed sarcoglycan have a certain effect on platelet accumulation. Platelets of wild-type, VWF and fibrinogen deficient, GPIBα-deficient, IL4Rα/ Gpibα-transgenic and αIIb deficient mice and humans were designed and obtained, and natural fucoglycan was hydrolyzed with hydrochloric acid. It was characterized by chromatography, UV-VIS spectroscopy, and fluorescence spectroscopy. Platelet activation markers (P-selectin expression, PAC-1, and fibrinogen binding) and platelet-VWFA1 interactions were detected by flow cytometry. The GPIbα-VWFA1 interaction was evaluated by enzyme-linked immunosorbent assay. western blot analysis of GPIb-ix induced signal transduction.
The amino acid residues EDRLPR in the ADAMTS13 domain were transfected into human embryonic kidney HEK293 cells by point mutation technique (mutants M1~M7), and the ability of wild-type and mutant ADAMTS13 to lysate VWF under denaturation conditions, shear stress, and ADAMTS13 antibody treatment was observed. The results showed that the VWF Western blotting method was used to detect wild-type and mutant ADAMTS13, and the Odyssey imaging system was used to analyze the relative molecular weight of wild-type and mutant ADAMTS13. Fluorescence resonance energy transfer (FRET) detection showed that ADAMTS13 mutant M4 (R635A) and mutant M7 (R638A) had a decreased shear ability to FRET-vWF73, indicating that there were multiple binding sites between the C-terminal of ADAMTS13 and vWF.
KMD Bioscience has been committed to the antibody field for many years and has a wealth of experience and mature technology. In addition, KMD Bioscience can also provide customized small molecule modification, accounting aptamer design, antibody expression, affinity determination, and antibody sequencing, antibodies have also made great achievements, etc to meet customer needs.
Related Products
| Cat# | Product Name | Species | Host | Applications | Size | Price |
| KMPH2468 | Human vWF Protein, His Tag | Human | CHO | 50ug, 100ug | Inquiry | |
| PAV509 | Rabbit Anti-ATS13/ADAMTS13 Polyclonal Antibody | Human | Rabbit | WB, ELISA | 100ul | Inquiry |
| PAV509 | Rabbit Anti-ATS13/ADAMTS13 Polyclonal Antibody | Human | Rabbit | WB, ELISA | 100ul | Inquiry |
| PAV4342 | Rabbit Anti-VWF Polyclonal Antibody | Human, Rat, Mouse | Rabbit | IHC-p | 100ul | Inquiry |
| MA438 | Rabbit Anti-Human VWF mAb | Human | Rabbit | 50ul, 100ul | Inquiry | |
| YR1265 | Anti-Human VWF A1 domain Recombinant Antibody | Human | CHO | 1mg, 5mg | Inquiry |
References:
[1] Zhu J, Wu Y, Yu Y, Li Y, Shen J, Zhang R. MYBL1 induces transcriptional activation of ANGPT2 to promote tumor angiogenesis and confer sorafenib resistance in human hepatocellular carcinoma [J]. Cell Death Dis. 2022, 13(8): 727.
[2] Karabid NM, Wiedemann T, Gulde S, et al. Angpt2/Tie2 autostimulatory loop controls tumorigenesis [J]. EMBO Mol Med. 2022, 14(5): e14364.
[3] Yang S, Zou X, Li J, et al. Immunoregulation and clinical significance of neutrophils/NETs-ANGPT2 in tumor microenvironment of gastric cancer [J]. Front Immunol. 2022, 13: 1010434.