2024-07-24 Hits(491)
Phage Display Technology
What is Phage Display Technology?
Phage display technology uses gene recombination to recombine the gene of the target protein into the gene of the phage capsid protein. Finally, it displays the target protein on the phage's surface as a fusion protein.
Application of Phage Display Technology
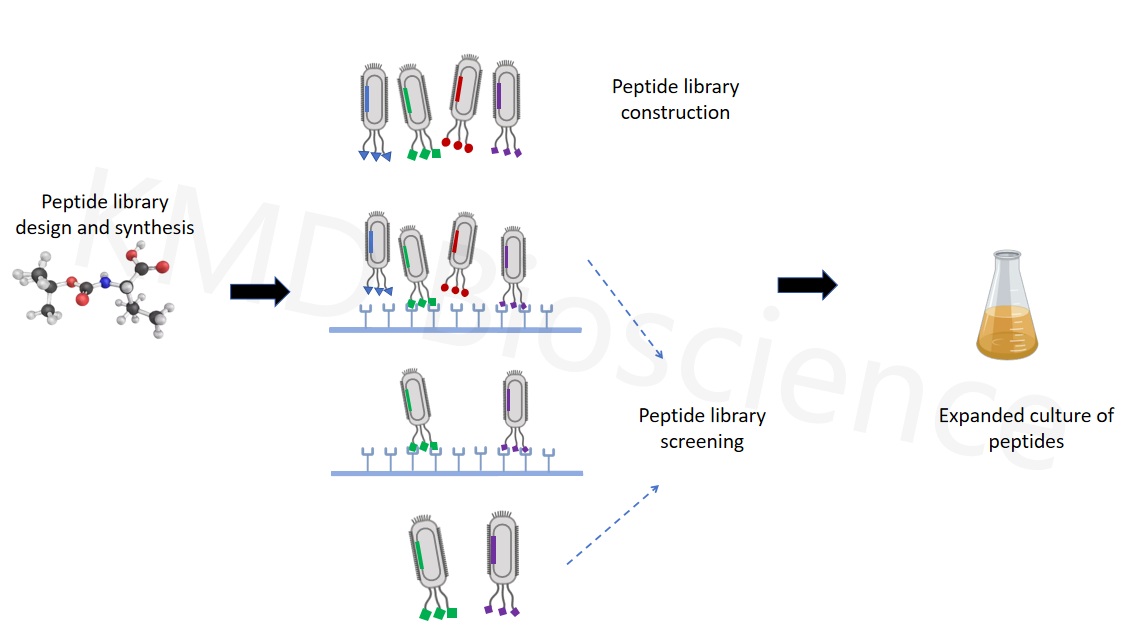
Peptides
Artificially synthesizing genes encoding random peptide segments, then cloning these genes into phage vectors, displaying peptides one by one on the surface of phages through phage display technology, and utilizing the ability of phages to replicate in large quantities to obtain multiple copies of different phages, constructing a diverse phage display peptide library. Phage peptide library can display the true epitopes of many protein peptides, and it is used to screen a very small number of peptides with key biological activities from a large number of peptides. Therefore, phage display technology plays an important role in the field of peptides. A large number of peptide drugs have been successfully developed through this technology. For example, Romiplostim, an idiopathic thrombocytopenic purpura treatment drug for the peptide drug Amegen that has already been marketed; Affiymax's anti-anemia drug - Pegine Satide; Dyax's Ecallantide.
Romiplostim
Romiplostim is a peptide fusion protein formed by screening peptide segments using phage display technology and connecting them to Fc fragments. It consists of two identical single-chain subunits, each with an IgG1 Fc vector binding domain that interacts with the thrombopoietin receptor (c-Mpl) to stimulate endogenous thrombopoietin production. Romistatin became the first platelet-derived drug used to treat thrombocytopenic purpura.
Ecanllantide
As a recombinant kinase-releasing enzyme protein inhibitor, Ecanlantinide was prepared through phage display technology and discovered from a phage display library constructed with Kunitz domain peptides (which can inhibit a large number of serine proteases at different intensities). Ecanlantinide is composed of 60 amino acids and can selectively and reversibly inhibit the activity of plasma kallikrein. The original manufacturer is Dyax.
Application of Phage Display Technology
Antibodies
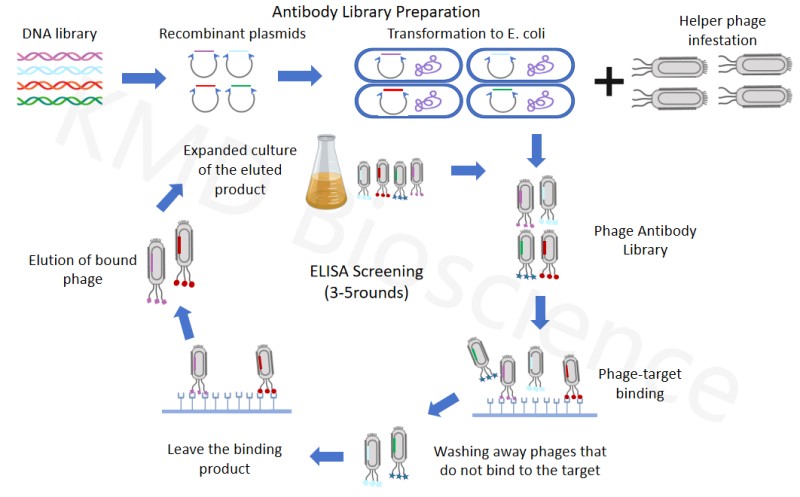
The fully human antibody is produced by phage display technology. The production workflow is to isolate human immune cells, amplify the entire set of antibody heavy chain variable region (VH) and light chain variable region (VL) genes from immune cells by PCR technology, integrate them into phage vectors, and express them in the form of fusion proteins on the surface of phages. Screening is performed using antigen antibody-specific binding and then transferred to host bacteria for cloning and amplification. Randomly combining VH and VL can create a combinatorial antibody library; If the antibody mRNA is derived from non-immunized normal individuals, a natural human antibody library can be established without the need for cell fusion, and the antibodies in the latter can prevent immune reactions in the human body.
Adalimumab
Adalimumab is the first fully human recombinant IgG1- κ monoclonal antibody developed using phage display technology, and also the world's first approved fully human monoclonal antibody against tumor necrosis factor-alpha (TNF - alpha). Adalimumab was obtained through "guided selection" screening using phage display technology. As a TNF antagonist, it can specifically bind to soluble human TNF - α and block its interaction with cell surface TNF - α receptors p55 and p75, effectively blocking the inflammatory effects of TNF - α and achieving the goal of treating diseases.
.png)
Belimumab
Belimumab is a human IgG1 λ monoclonal antibody with a relative molecular weight of 147000. It can specifically bind soluble B cell stimulating factor (BLyS), prevent BLyS from binding to B cells, promote B cell apoptosis, and achieve the goal of treating SLE. The discovery of belimumab is attributed to the combination of phage library technology and high-throughput sequencing technology. CAT screened single chain variable fragments (scFv) that bind to human BlyS through a phage library, and identified 1200 antibodies; HSG utilized its high-throughput sequencing technology to determine the sequence of each antibody's heavy and light chains, ultimately obtaining the high-affinity belimumab in 2003.