2024-06-05 Hits(141)
DNA Extraction
Plasmid isolation, also known as plasmid purification, is a routine procedure used to extract and purify plasmid DNA from bacterial cells. Here is a detailed protocol for plasmid isolation using a commonly employed alkaline lysis method.
Materials and Reagents
| Bacterial Culture | E. coli containing plasmid | / |
| Buffer Solutions | Solution I (Resuspension Buffer) |
50 mM Tris-HCl (pH 8.0); 10 mM EDTA; 100 µg/mL RNase A |
| Solution II (Lysis Buffer) |
200 mM NaOH; 1% SDS |
|
| Solution III (Neutralization Buffer) | 3 M Potassium acetate (pH 5.5) | |
| Solution IV (Equilibration Buffer) |
750 mM NaCl; 50 Mm MOPS, PH 7.0; 15% isopropanol |
|
| Solution V ( Elution Buffer) |
1.23 M NaCl; 50 mm Tris-Cl, pH 8.5; 15% isopropanol |
|
| TE Buffer | 10 mM Tris-HCl (pH 8.0), 1 mM EDTA | |
| Other |
Isopropanol; Ethanol (70%); Centrifuge; Vortex Mixer |
Centrifuge Tubes: 1.5 mL; Microcentrifuge Tubes; Pipettes and Tips |
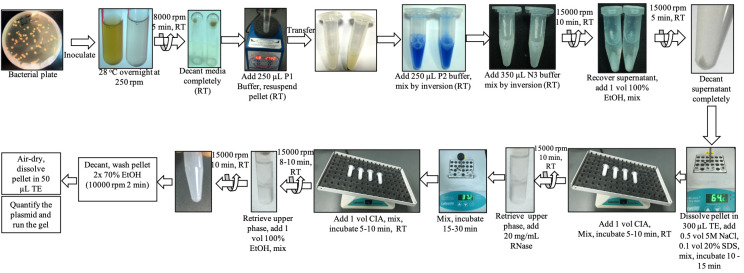
(Figure Source: doi: 10.21769/BioProtoc.4788.)
Plasmid DNA Extraction Process
Bacterial Culture Preparation:
Choose a single colony of E. coli containing the plasmid from a freshly streaked selective plate and inoculate a starter culture of 2–5 ml LB medium containing the appropriate selective antibiotic. Dilute the starter culture 1/500 to 1/1000 into 3 ml selective LB medium. Incubate the culture overnight at 37°C with vigorous shaking (200-250 rpm).
Cell Harvesting:
Transfer 1.5 mL of the overnight culture into a 1.5 mL microcentrifuge tube. Centrifuge at 12,000 rpm (≈13,000 x g) for 1 minute (or: 6000 x g for 15 min) at room temperature to pellet the bacterial cells. Discard the supernatant carefully, and remove as much of the supernatant as possible
Cell Resuspension:
Add 100 µL to 500 µL of Solution I to the cell pellet. The bacteria should be resuspended completely by vortexing or pipetting up and down until no cell clumps remain.
Cell Lysis:
Add 200 µL of Solution II to the resuspended cells. Mix gently by inverting the tube 4-6 times. Do not vortex as this can shear the DNA. Incubate at room temperature for 5 minutes. The solution should become clear and viscous. Do not allow the lysis reaction to proceed for more than 5 min.
Neutralization:
Add 300 µL of Solution III to the lysate, mix immediately and thoroughly by inverting the tube 4-6 times, and incubate on ice for 5 min. White precipitates should form. Centrifuge at 12,000 rpm for 10 minutes at room temperature. A fluffy white material forms after adding a neutralization buffer, and the lysate becomes less viscous. The precipitated material contains genomic DNA, proteins, cell debris, and KDS. The lysate should be mixed thoroughly to ensure even potassium dodecyl sulfate precipitation. If the mixture still appears viscous, more solution III is required to neutralize the solution completely. A homogeneous colorless suspension indicates that the SDS has been effectively precipitated.
Load Lysate on Column
Before loading the column, carefully remove the supernatant and then transfer it to a collection tube containing the column and centrifuge at 13,000 rpm for 1 minute. Discard the flow-through liquid and remove the supernatant containing plasmid DNA promptly. After centrifugation, the supernatants should be clear. If the supernatant is not clear, a second, shorter centrifugation should be carried out to avoid applying any suspended or particulate material to the column. Suspended material (which causes the sample to appear turbid) will clog the column and reduce or eliminate flow.
Bind and Wash
Add 0.7 ml of wash buffer to the column placed in the collection tube and centrifuge for 10 minutes at 13000 rpm for 1 minute. Equilibrate by applying 1 ml equilibration buffer ( 750 mM NaCl, 50 Mm MOPS, ph 7.0, 15 % isopropanol ) and allow the column to empty by gravity flow. The flow of buffer will begin automatically with a reduction in surface tension due to the presence of detergent in the equilibration buffer.
Plasmid DNA Precipitation:
Elute DNA with 0.8 ml solution V, and collect the elute in a 1.5 ml or 2 ml microcentrifuge tube. Precipitate DNA by adding 0.7 volumes (0.56 ml per 0.8 ml of solution V volume) of room-temperature isopropanol to the eluted DNA. Centrifuge at 12,000 rpm for 10 minutes at room temperature to pellet the plasmid DNA. Mix and centrifuge immediately at ≥10,000 rpm for 30 min in a microcentrifuge. Carefully decant the supernatant. All solutions should be at room temperature to minimize salt precipitation.
Wash DNA Pellet:
Carefully discard the supernatant, and add 1 mL of 70% ethanol to wash the DNA pellet. Centrifuge at 12,000 rpm for 5 minutes at room temperature. Carefully discard the supernatant without disturbing the pelle t.The 70% ethanol removes precipitated salt and replaces isopropanol with the more volatile ethanol, making the DNA easier to redissolve.
Dry and Resuspend DNA:
Air-dry the DNA pellet for 5-10 minutes to remove residual ethanol. Avoid overdrying the pellet as this can make it difficult to dissolve. Resuspend the DNA pellet by rinsing the walls to recover all the DNA in 50 µL of TE buffer or sterile distilled water.
Determination of Yield
To determine the yield, DNA concentration should be determined by both UV spectrophotometry at 260 nm and quantitative analysis on an agarose gel. To quantitate the nucleic acid concentration, dilute the plasmid DNA 1: 100 or 1: 50 (depending on the plasmid copy number) in TE buffer and measure the absorbance (optical density) at 260 nm (A260) and 280 nm (A280). Use TE buffer as the blank. This measurement permits the direct calculation of the nucleic acid concentration using the formula.
[DNA] (μg/mL) = A260 × Dilution factor × 50
where 50 is the extinction coefficient of DNA. This measurement method can directly calculate the nucleic acid concentration using a formula.
Storage and Use
The isolated plasmid DNA can be stored at -20°C for long-term storage. Use the purified plasmid DNA for downstream applications such as cloning, PCR, restriction digestion, or sequencing.
Notes
- Ensure that all solutions are prepared freshly and that all reagents are of molecular biology grade.
- Avoid excessive vortexing to prevent shearing of plasmid DNA.
-Confirm the concentration and purity of the plasmid DNA using spectrophotometry or gel electrophoresis.