2024-06-13 Hits(134)
Antibody Discovery
Antibodies, known as immunoglobulins (Igs), can recognize and clear specific foreign objects in the body. Due to its natural ability to bind to target antigens, antibodies have shown enormous value in biomedical and drug development. The production of monoclonal antibodies is aimed at improving antigen recognition specificity, eliminating immunogenicity, and enhancing the affinity and stability of antibodies.
In 1993, a natural small functional antibody called heavy chain antibody (HCAb) was first reported in camel serum. Unlike traditional antibodies with hetero-tetragonal structures, camel-derived HCAb lacks light chain peptides and a first constant domain (CH1) in the heavy chain. The antigen binding fragment in HCAbs only contains a single variable domain. This structural domain is called VHH, also known as single domain antibody (sdAb) or nanobody (Nb). Single-domain antibodies originate from the Camelidae family, such as llamas, camels, and alpacas. Single domain antibodies with a molecular weight of only 12-15 kDa are much smaller than traditional antibodies composed of two heavy chains and two light chains (150-160 kDa), and even smaller than Fab fragments (50 kDa, one light chain and half heavy chain) and single chain variable antibody fragments (25 kDa, two variable structural domains, one light chain, and one heavy chain), and have higher affinity and stability. Single-domain antibodies are being studied for multiple drug applications and may be used to treat acute coronary syndrome, cancer, Alzheimer's disease, and COVID-19.
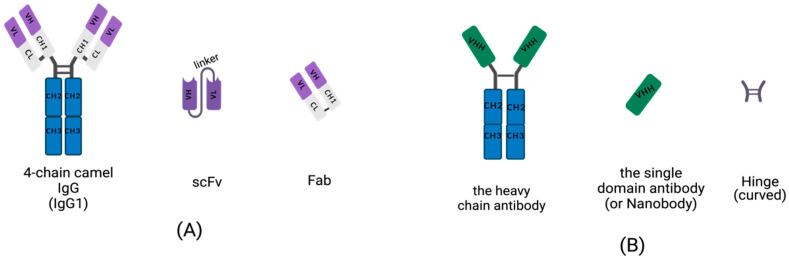
Figure Source: doi: 10.3390/ijms24044176
Properties of Single Domain Antibody
Single-domain antibodies have many advantages in biotechnology applications. The nanobody libraries produced by immune camels and alpacas retain complete diversity. By binding high-affinity antigens to nano antibodies, a limited number of clones can be directly screened from the immune library for isolation, without the need for phage display technology for pre-screening. Single-domain antibodies have high stability and still function at high temperatures. It can recognize antigen sites that are usually not recognized by conventional antibodies, and due to its small size of about 15 kDa, it can perform rapid tissue penetration. Antibodies derived from camel and fish have lower lipophilicity and are more soluble in water.
Single Domain Antibody Production
Completely functional antibodies are efficiently produced in mammalian cells only, and appropriate glycosylation of these antibodies is extremely important for exerting the required therapeutic activity. However, microbial production systems such as Escherichia coli (E. coli), filamentous fungi, or yeasts are used for large-scale and economic production of antibodies.
VHH can generally be produced well in microorganisms but the production level depends on the VHH sequence patterns. Sagt and his colleagues have reported an increase in the production of VHH in yeast due to the presence of a potential N-linked glycosylation site.
Moreover, studies on baker’s yeast have demonstrated that the production of VHH in yeast can be increased up to fivefold by the addition of ethanol and supplementing the growth medium with ethylenediaminetetraacetic acid (EDTA), sorbitol, or casaminoacids.
In yet another research study, there was a reduction in the production of VHH due to the presence of unpaired C-terminal cysteines. DNA shuffling also enhances VHH production by random molecular evolution.
In conclusion, camelid VHHs are suitable for a variety of therapeutic applications, e.g. in sleeping sickness, infant diarrhea caused by rotavirus, foot-and-mouth disease, sepsis, rheumatoid arthritis, brain disorders, neurodegenerative diseases, etc. VHHs are especially effective as oral immunotherapy in diarrhea due to their ability to withstand extreme pH values.