2025-04-11 Hits(51)
TCR-T Cell Therapy
Definition of TCR Cell Therapy
TCR cell therapy, or T Cell Receptor (TCR) therapy, is a form of adoptive cell therapy that involves genetically engineering a patient’s T cells to express a specific T cell receptor (TCR) that recognizes and targets cancer cells. Unlike CAR-T cell therapy, which uses a synthetic Chimeric Antigen Receptor (CAR) to recognize surface antigens, TCR therapy leverages the natural ability of T cells to recognize intracellular antigens presented by the Major Histocompatibility Complex (MHC) on the surface of cells.
Key Components of TCR Cell Therapy
(1) T Cell Receptor (TCR):
A naturally occurring receptor on T cells that recognizes peptide fragments (antigens) presented by MHC molecules. The TCR is composed of alpha and beta chains (or gamma and delta chains in some cases).
(2) Target Antigen:
TCR therapy targets intracellular antigens (e.g., cancer-specific proteins or viral proteins) that are processed and presented by MHC molecules.
(3) MHC Restriction:
TCRs recognize antigens only when they are presented by specific MHC molecules, making TCR therapy MHC-dependent.
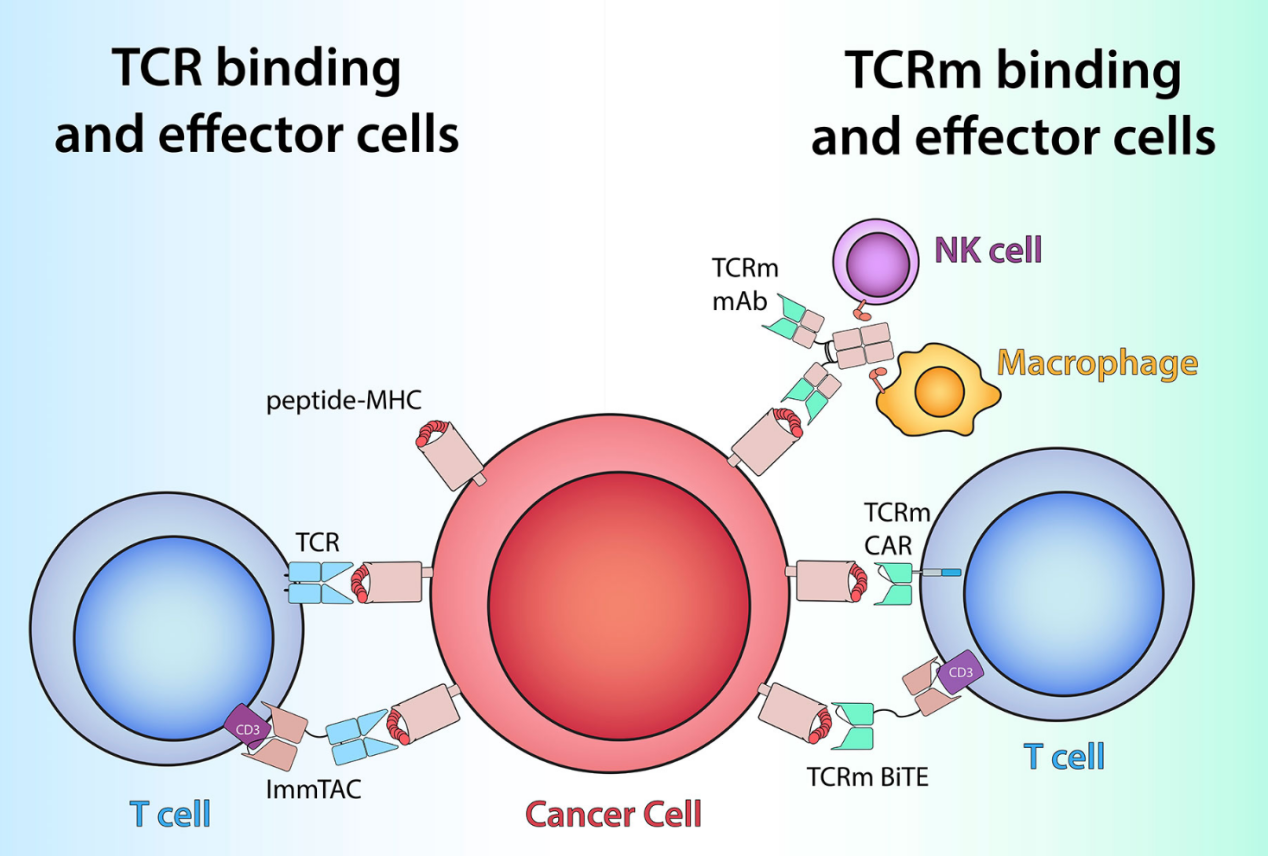
Figure 1:TCR-based therapeutics recognize peptide/MHC antigens (red and pink) on cells by utilizing either TCRs (light blue) or TCRm antigen-binding domains (green)[1]
TCR Cell Mechanism
TCR (T Cell Receptor) cell therapy is a form of adoptive cell therapy that involves genetically engineering a patient’s T cells to express a specific T cell receptor (TCR) that can recognize and target cancer cells. The mechanism of TCR cell therapy relies on the natural ability of T cells to recognize antigens presented by the Major Histocompatibility Complex (MHC) on the surface of cells. Here’s a detailed breakdown of the mechanism:
(1) T Cell Collection
Process: T cells are extracted from the patient’s blood through a procedure called leukapheresis.
Purpose: These T cells will be genetically modified to express a TCR that targets a specific cancer-associated antigen.
(2) Genetic Engineering
TCR Design: A TCR is selected or engineered to recognize a specific peptide-MHC complex on cancer cells. The TCR is composed of alpha and beta chains (or gamma and delta chains in some cases).
Gene Delivery: The TCR gene is introduced into the T cells using viral vectors (e.g., retrovirus or lentivirus) or non-viral methods like electroporation. The genetically modified T cells now express the desired TCR on their surface.
(3) T Cell Expansion
Process: The engineered TCR-T cells are cultured and expanded in the lab to produce a large population of cancer-fighting cells.
Purpose: Ensures there are enough TCR-T cells to effectively target and destroy cancer cells in the patient.
(4) Infusion into the Patient
Process: The expanded TCR-T cells are infused back into the patient’s bloodstream.
Purpose: The TCR-T cells circulate throughout the body, seeking out and attacking cancer cells.
(5) Target Recognition and Binding
Process: The TCR on the engineered T cells recognizes a specific peptide-MHC complex on the surface of cancer cells. The peptide is derived from an intracellular protein (e.g., a cancer-associated antigen) that is processed and presented by the MHC molecule.
(6) T Cell Activation
Process: Upon binding to the peptide-MHC complex, the TCR initiates a signaling cascade through the CD3 complex and co-stimulatory molecules (e.g., CD28). This activates the T cell, leading to proliferation, cytokine secretion, and cytotoxic activity.
Key Components: CD3 Complex: Transduces the activation signal from the TCR. Co-stimulatory Molecules: Enhance T cell activation and persistence.
(7) Cancer Cell Destruction
Cytotoxicity: Activated TCR-T cells release cytotoxic molecules like perforin and granzymes, which puncture and kill cancer cells.
Cytokine Release: TCR-T cells secrete cytokines (e.g., IFN-γ, IL-2) that enhance the immune response and recruit other immune cells.
Proliferation: TCR-T cells multiply in the body, increasing their numbers and amplifying the anti-cancer effect.
(8) Persistence and Memory
Long-Term Activity: TCR-T cells can persist in the body for months or even years, providing ongoing surveillance against cancer recurrence.
Memory Formation: Some TCR-T cells differentiate into memory T cells, which can quickly respond if the cancer returns.
TCR Cell Therapy Process
TCR (T Cell Receptor) cell therapy is a sophisticated form of adoptive cell therapy that involves genetically engineering a patient’s T cells to express a specific T cell receptor (TCR) capable of recognizing and targeting cancer cells[3]. The process is highly personalized and involves several key steps, from T cell collection to infusion and monitoring. Here’s a detailed breakdown of the TCR cell therapy process:
(1) Patient Evaluation and Eligibility
Purpose: Determine if the patient is a suitable candidate for TCR therapy.
Criteria: Diagnosis of a cancer type that expresses a targetable antigen (e.g., melanoma, sarcoma, or certain blood cancers). Expression of the appropriate MHC molecule to present the target antigen. Failure of standard treatments like chemotherapy, radiation, or immunotherapy.
(2) T Cell Collection (Leukapheresis)
Process: The patient’s blood is drawn and passed through a machine that separates T cells from other blood components. The collected T cells are sent to a specialized laboratory for genetic modification.
Duration: 3–6 hours.
(3) Genetic Engineering of T Cells
Step 1: TCR Design: A TCR is selected or engineered to recognize a specific peptide-MHC complex on cancer cells. The TCR is composed of alpha and beta chains (or gamma and delta chains in some cases).
Step 2: Gene Delivery: The TCR gene is introduced into the T cells using viral vectors (e.g., retrovirus or lentivirus) or non-viral methods like electroporation. The genetically modified T cells now express the desired TCR on their surface.
(4) T Cell Expansion
Process: The engineered TCR-T cells are cultured in the lab and stimulated to multiply, creating a large population of cancer-fighting cells.
Duration: 2–3 weeks.
(5) Lymphodepletion (Preconditioning)
Purpose: Prepare the patient’s body to receive the TCR-T cells.
Process: The patient undergoes chemotherapy (e.g., fludarabine and cyclophosphamide) to deplete existing immune cells. This creates space and reduces competition for the infused TCR-T cells, enhancing their effectiveness.
Duration: 3–5 days before TCR-T cell infusion.
(6) TCR-T Cell Infusion
Process: The expanded TCR-T cells are infused back into the patient’s bloodstream through an intravenous (IV) line. The TCR-T cells circulate throughout the body, seeking out and attacking cancer cells.
Duration: 30 minutes to a few hours.
(7) Monitoring and Management of Side Effects
Purpose: Monitor the patient for side effects and provide supportive care.
Common Side Effects:
① Cytokine Release Syndrome (CRS):
Symptoms: Fever, low blood pressure, difficulty breathing.
Treatment: Tocilizumab (an IL-6 receptor antagonist) and corticosteroids.
② Neurotoxicity:
Symptoms: Confusion, seizures, speech difficulties.
Treatment: Supportive care and corticosteroids.
③ On-Target, Off-Tumor Toxicity:
Symptoms: Damage to healthy cells expressing the target antigen.
Treatment: Close monitoring and symptom management.
Duration: Patients are closely monitored for several weeks after infusion.
(8) Long-Term Follow-Up
Purpose: Assess the effectiveness of the therapy and monitor for long-term side effects or cancer recurrence.
Process: Regular blood tests, imaging, and clinical evaluations. Monitoring for late-onset side effects or immune-related complications.
TCR vs CAR-T
TCR (T Cell Receptor) therapy and CAR-T (Chimeric Antigen Receptor T-cell) therapy are both forms of adoptive cell therapy that involve genetically engineering a patient’s T cells to target and destroy cancer cells. However, they differ in their mechanisms, targets, advantages, and limitations.
(1) Mechanism of Action
|
Feature |
TCR Therapy |
CAR-T Therapy |
|
Recognition |
Recognizes peptide-MHC complexes. |
Recognizes surface antigens directly. |
|
Target Antigens |
Intracellular antigens presented by MHC. |
Surface antigens (e.g., CD19, BCMA). |
|
MHC Restriction |
Yes (MHC-dependent). |
No (MHC-independent). |
|
Signaling Pathway |
Uses natural TCR signaling (CD3 complex). |
Uses synthetic CAR signaling (CD3ζ + co-stimulatory domains). |
(2) Target Antigens
|
Feature |
TCR Therapy |
CAR-T Therapy |
|
Antigen Type |
Intracellular proteins processed and presented by MHC. |
Surface proteins (e.g., CD19, CD20, BCMA). |
|
Examples |
NY-ESO-1, MART-1, WT1. |
CD19 (B-cell cancers), BCMA (multiple myeloma). |
(3) Applicability
|
Feature |
TCR Therapy |
CAR-T Therapy |
|
Cancer Types |
Broad (solid tumors and blood cancers). |
Primarily blood cancers (e.g., ALL, DLBCL). |
|
Solid Tumors |
Effective for some solid tumors (e.g., melanoma, sarcoma). |
Limited efficacy in solid tumors. |
|
Blood Cancers |
Can target blood cancers (e.g., leukemia). |
Highly effective for blood cancers. |
(4) Advantages
|
Feature |
TCR Therapy |
CAR-T Therapy |
|
Target Range |
Can target intracellular antigens. |
Limited to surface antigens. |
|
Specificity |
High specificity for peptide-MHC complexes. |
High specificity for surface antigens. |
|
Natural T Cell Function |
Utilizes natural TCR signaling pathways. |
Uses synthetic CAR signaling. |
|
Broad Applicability |
Effective for solid tumors and blood cancers. |
Highly effective for blood cancers. |
TCR Therapy: Best suited for targeting intracellular antigens and treating solid tumors, but limited by MHC restriction and potential off-target effects.
CAR-T Therapy: Highly effective for blood cancers, with no MHC restriction, but limited to surface antigens and associated with severe side effects.
Both therapies represent groundbreaking advances in cancer treatment, and the choice between them depends on the type of cancer, target antigen, and patient-specific factors. Ongoing research aims to overcome their limitations and expand their applications.
Application of TCR Cell Therapy
TCR (T Cell Receptor) cell therapy is a promising form of adoptive cell therapy that involves genetically engineering a patient’s T cells to express a specific T cell receptor (TCR) capable of recognizing and targeting cancer cells. Unlike CAR-T therapy, which targets surface antigens, TCR therapy leverages the natural ability of T cells to recognize intracellular antigens presented by the Major Histocompatibility Complex (MHC). Here are the key applications of TCR cell therapy:
(1) Cancer Treatment
TCR therapy has shown significant potential in treating various types of cancers, particularly solid tumors, by targeting intracellular antigens.
(2) Viral Infections
TCR therapy is being developed to target viral infections by recognizing viral antigens presented by MHC molecules.
① HIV:
Target Antigens: HIV-specific peptides.
Outcomes: TCR therapy aims to eliminate HIV-infected cells.
② Hepatitis B Virus (HBV):
Target Antigens: HBV-specific peptides.
Outcomes: TCR therapy is being explored to treat chronic HBV infection.
(3) Autoimmune Diseases
TCR therapy is being investigated as a potential treatment for autoimmune diseases by targeting autoreactive T cells or B cells.
TCR cell therapy is a versatile and powerful approach to cancer treatment, particularly for solid tumors, by targeting intracellular antigens presented by MHC molecules. Its applications are expanding to include viral infections and autoimmune diseases, offering hope for patients with limited treatment options. While challenges like MHC restriction and off-target effects remain, ongoing research and innovation are improving the safety, efficacy, and applicability of TCR therapy, making it a valuable addition to the arsenal of immunotherapies.
References
[1] Pagel JM, West H. Chimeric Antigen Receptor (CAR) T-Cell Therapy. JAMA Oncol. 2017;3(11):1595. doi:10.1001/jamaoncol.2017.2989
[2] Regan C , Clark J , Moore T ,et al.T-cell therapy in metastatic melanoma: TIL 1383I TCR transduced T cells after infusion and activity in vivo.[J].Journal of Clinical Oncology, 2015, 33(15_suppl):3043-3043.DOI:10.1200/jco.2015.33.15_suppl.3043.
[3] Weinberg A , Celnik B , Vainiene M ,et al.The effect of TCR Vβ8 peptide protection and therapy on T cell populations isolated from the spinal cords of Lewis rats with experimental autoimmune encephalomyelitis[J].Journal of Neuroimmunology, 1994, 49:161-170.DOI:10.1016/0165-5728(94)90192-9.