2024-12-12 Hits(36)
KMD Bioscience's research team boasts extensive expertise in antibody production, having developed a comprehensive and efficient research and development system for antibodies. Over the years, KMD Bioscience has set up a holistic antibody technology platform, encompassing a full suite of upstream and downstream services—ranging from immunogenicity preparation, animal immunization, antibody discovery, recombinant antibody expression verification, to polyclonal antibody purification support. Additionally, the platform offers diverse antibody preparation services, which extend to bispecific antibody development, efficient blocking neutralizing antibody creation, antibody humanization, drug target antibody generation, and ADC (antibody-drug conjugate) development, among others. Leveraging standardized production lines, KMD Bioscience provides customers with neutralizing polyclonal antibody preparation services tailored for viral antigens, complemented by services such as antibody humanization, affinity maturation, ADC drug design and development, and CAR-T sequence design. These services ensure rigorous adherence to quality standards throughout each experimental step, yielding high-quality neutralizing antibodies.
For virus samples, KMD Bioscience can provide a wide range of animal derived neutralizing polyclonal antibody preparation services (including rabbit, mouse, camel, sheep, chicken, etc.). At the same time, KMD Bioscience can also customize immunogens according to customer needs, with professional technicians designing, synthesizing, and packaging viruses. After immunization, the ELISA titer of polyclonal antibodies can reach 1:50000 or more. Animals such as cows and sheep with larger body sizes have higher antibody titers prepared after immunization, and their ELISA titers can reach 10^5. KMD Bioscience verifies the expression of virus immunogens prepared through WB validation, and verifies serum antibody titers through ELISA and FACS to ensure reliable results, saving customers time and reducing experimental costs.
Simultaneously, KMD Bioscience offers customers high-quality, species-specific polyclonal antibody production services through its advanced antibody technology and extensive production process. Within a shortened preparation timeframe, a comprehensive array of customized services is available, encompassing antigen design and synthesis, animal immunization, as well as antibody purification and identification. These services cater to the personalized needs of customers' scientific research projects.
Neutralization Polyclonal Antibody Discovery Service
Polyclonal antibodies can recognize multiple epitopes on the same antigen and have the advantages of short preparation cycle and low cost. When a virus invades the body, the B lymphocytes of the body produce neutralizing antibodies, which have antiviral activity and can recognize and bind to specific antigenic epitopes on the virus surface, effectively preventing the virus from binding to host cells, thereby blocking its infection pathway and replication process. Neutralizing polyclonal antibodies have high affinity and can recognize multiple epitopes on antigens, thereby enhancing their binding ability to antigens and exhibiting high affinity. This high affinity enables polyclonal antibodies to achieve better results in experiments such as immunoprecipitation. Polyclonal antibodies have stronger tolerance for changes in small antigens, such as polymorphism, glycosylation heterogeneity, or mild denaturation, and can therefore recognize proteins with high homology to immunogenic proteins, and can even be used to screen target proteins of non immunogenic species. The role of neutralizing polyclonal antibodies is more comprehensive, with important functions such as neutralizing antigens, immune regulation, mediating complement dependent cytotoxicity, and antibody dependent cell-mediated cytotoxicity. Neutralizing polyclonal antibodies have a wide range of sources and are relatively easy to prepare. Polyclonal antibodies can be obtained through animal immune serum, with a wide range of sources and relatively easy preparation. Neutralizing polyclonal antibodies have wide applications in biomedical research, such as protein function studies, cell signaling studies, and exploration of disease pathogenesis. They can recognize multiple antigenic epitopes, providing rich information for research. Polyclonal antibodies are also commonly used in clinical diagnosis, such as diagnosis of infectious diseases, detection of tumor markers and diagnosis of autoimmune diseases. Their high affinity and inclusiveness make diagnostic results more accurate and reliable. By using specific neutralizing polyclonal antibodies against pathogens in vitro, the ability of pathogens to invade the human body can be directly inhibited, thereby preventing infection. For example, during the epidemic of COVID-19, researchers found that the neutralizing inhibition rate of IgY neutralizing antibody (a polyclonal antibody) extracted from the egg yolk of immunized hens to a variety of COVID-19 variants was more than 98%, showing its potential in passive immunotherapy. Neutralizing polyclonal antibodies have broad application prospects in biomedical research, clinical diagnosis, and passive immunotherapy due to their high affinity, strong inclusiveness, comprehensive effects, wide sources, and easy preparation.
Antibody neutralization test is a process in which a virus or toxin is mixed and incubated with a corresponding specific antibody under appropriate conditions in vitro, allowing the virus or toxin to react with the antibody. Then, the mixture is inoculated into sensitive hosts (such as chicken embryos, cultured cells, or experimental animals), and the infectivity of residual viruses or toxins is determined. The basic principle of antibody neutralization test is that specific antiviral antibodies (neutralizing antibodies) can bind to viruses or toxins, block virus surface antigens, bind to virus surface adsorption proteins, and thus block the virus's ability to adsorb and penetrate host cells. In this way, the replication of the virus within the host cell and the process of infecting the body are prevented.
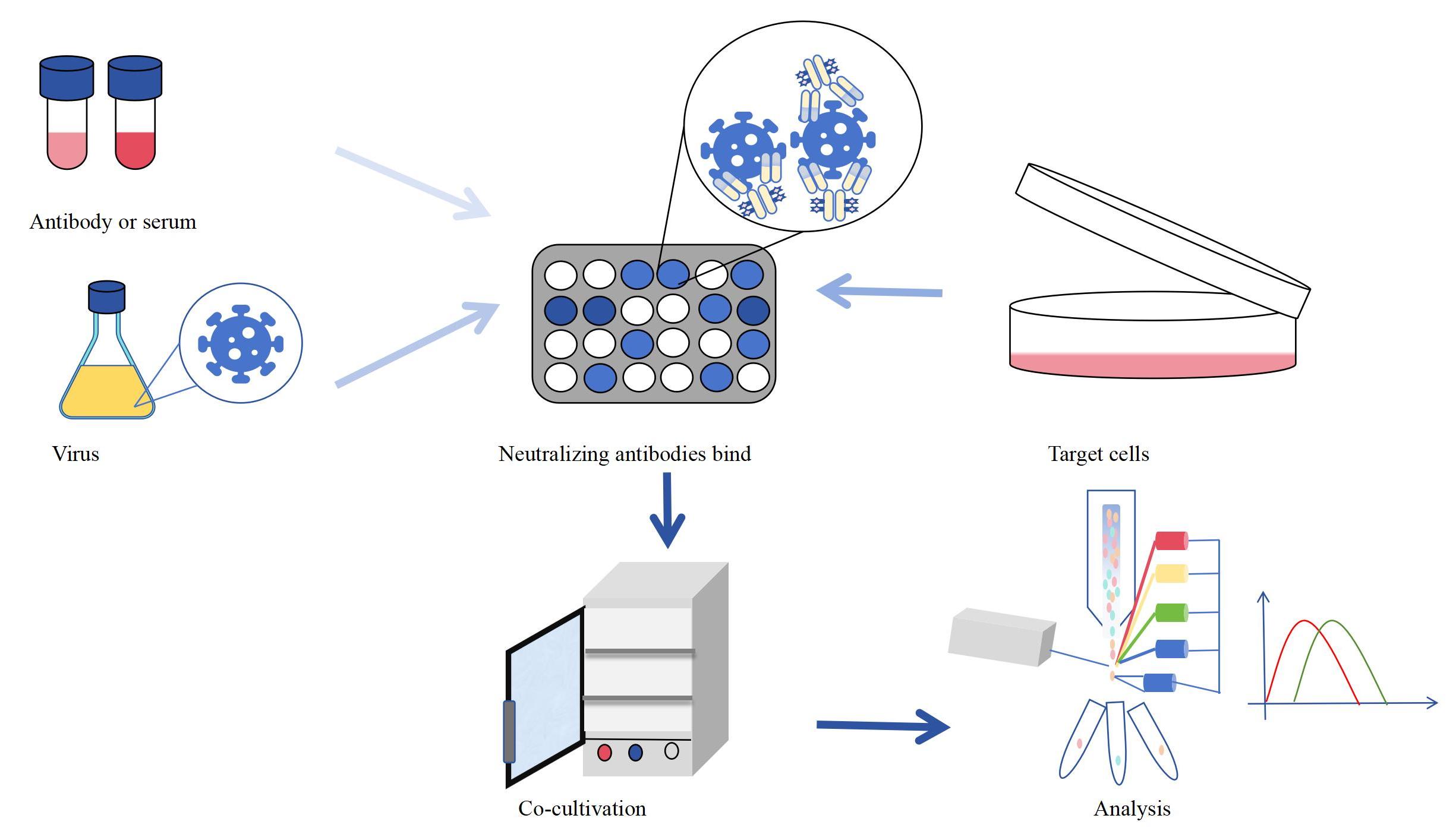
Figure 1: Antibody neutralization test process
The neutralizing antibodies prepared by KMD Bioscience have strong specificity and almost no-cross reactivity. According to the customer's needs, virus antigens are used to immunize mice, and the potency is tested by ELISA to be qualified. The neutralizing effect of the prepared antibodies on the virus can be evaluated through antibody neutralization test. Afterwards, the antibodies are purified. KMD Bioscience has multiple antibody purification equipment and can provide affinity purification services and antibody separation and purification services to ensure the purity and quality of the delivered antibodies. Figure 2 shows a schematic representation of possible neutralization mechanisms using the interaction of SARS-CoV-2 with its receptors as an example.
.jpg)
Figure 2 Interaction of SARS-CoV-2 with its receptors and neutralization mechanisms. (Figure Source: Morales-Núñez JJ, Muñoz-Valle JF, et,al,. Overview of Neutralizing Antibodies and Their Potential in COVID-19. Vaccines. )
Neutralization Polyclonal Antibody Discovery Service Workflow
|
Step |
Service Content |
Timeline |
|
Step 1: Animal immunity |
(1) The animal was immunized 4 times, with one booster shot, for a total of 5 doses. (2) Negative serum before immunization was collected, and ELISA was performed on the fourth dose to detect serum titer. (3) If the fourth dose of serum antibody titer meets the requirements, booster immunization will be administered again 7 days before blood collection. If it does not meet the requirements, routine immunization will continue. (4) Qualified potency, blood collection and separation of monocytes. |
10 weeks |
|
Step2: Neutrality test |
(1) Co-incubation of neutralizing antibodies and viruses. (2) The mixture is inoculated into the host body (such as animals, chicken embryos, cells). (3) The infectivity of residual viruses has been determined. |
4-5 weeks |
|
Step3: Antibody purification |
Antibody Purification and Validation |
3-4 weeks |
Advantages of Neutralization Polyclonal Antibody Discovery Service
.jpg)
FAQ-Neutralization Polyclonal Antibody Discovery
1. What are the neutralizing polyclonal antibodies?
A: After the virus invades the body, it will stimulate the body to produce antibodies, which are transported to the whole body with the blood and play a protective role in the body. In this process, the antibodies produced by the body are divided into neutralizing antibodies and non-neutralizing antibodies. Neutralizing antibody can bind to the antigen of the virus, so that the virus can not adsorb to cells, damage cells, and have a protective effect on the body. Neutralizing antibodies can be used as antiviral agents for prophylaxis and treatment and contribute in viral vaccine design.There are two common types of neutralizing antibodies, monoclonal antibodies, and polyclonal antibodies, both of which have their advantages and disadvantages.The polyclonal antibody preparation took a short time, requiring only collection of immunized animal serum and no other subsequent steps such as hybridoma cell preparation and detection.MAbs can only target one antigenic site, while multiple antibodies can recognize various sites. However, compared with monoclonal antibodies, the poly antibody composition is complex and easy to bind to other substances, while the monoclonal antibody does not bind to other substances, so the recognition site is relatively single and has higher specificity.
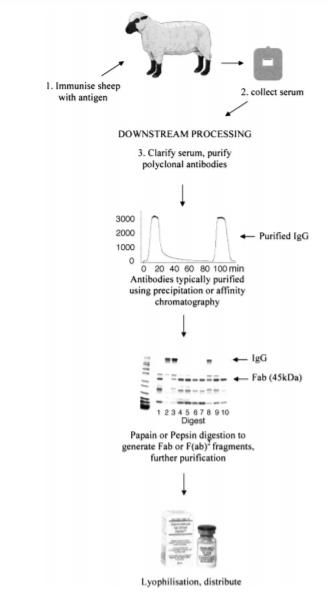
Figure 3 Procedures for the preparation of neutralizing polyclonal antibodies. (Reference source: Newcombe C, Newcombe AR. Antibody production: polyclonal-derived biotherapeutics. J Chromatogr B Analyt Technol Biomed Life Sci. 2007 Mar 15;848(1):2-7.)
2. In the neutralizing polyclonal antibody development service, how to screen and identify immunogens, and how to purify antibodies simultaneously?
A: When selecting immunogens, we first consider the purity, immunogenicity, presence and stability of the antigen. Generally speaking, the high immunogenicity of the antigen is easy to cause the immune response of the body. At the same time, we also consider whether the structure of the antigen itself affects the antibody preparation. The immunogens we prepared neutralizing polyclonal antibodies can generally be proteins, viruses, etc.We had better choose the purified virus, which can reduce the influence of irrelevant impurities such as cells and improve the specificity of serum.Small molecular substances we generally through the coupling, increase the molecular weight, strengthen the immunogenicity of the antigen. After the success of neutralizing polyclonal antibodies, we used affinity chromatography and molecular sieve to reduce the effects of irrelevant proteins and factors in serum. The preparation step of multi-antibody preparation is complicated, involving many links such as antigen preparation, immune dose, immune mode and cycle.It has a powerful protein platform in neutralization polyclonal antibody customization services, which can ensure the high potency of our products and high purity of neutralizing antibodies. At the same time, it can also provide a variety of different antibody customization services, such as small molecule multi-anti-customization, to meet the specific needs of different customers.
3. In the neutralizing polyclonal antibody development service, how do we detect multi-antiquity methods? If the antibody quality is not good, how do we solve it?
A: The specificity, potency, affinity, and cross-reactivity of polyantibodies were tested. The specific reaction is the ability of the antigen to bind the antibody, potency indicates the content of the antibody obtained, affinity is the strength of the antibody and antigen binding, and cross reactivity is whether the antibody can work with other substances. Specificity can determine the ability of the antibody to bind to the target antigen without recognizing the non-target antigen, which reflects the accurate recognition of the antigen by the antibody. Potency, also known as antibody titer, can make a judgment of the antibody content obtained by us, which is related to the biological activity of the antibody itself. Affinity shows the closeness and stability between the antibody and the antigen, while high affinity means that a stable complex is formed between the antibody and the antigen. Cross-reactivity is to see if the antibody can bind to other antigens.If the antibody quality is poor, we can first optimize our antigens. At the same time, we can also replace different immune adjuvants. In addition to the Freund's complete and incomplete adjuvants often used, we can also use other new adjuvants to try to increase immunogenicity. At the same time, our immunization program should be redesigned according to the different immunogens and adjuvants to improve the potency. At the same time, we can take multi-point back injections, peritoneal injections, intramuscular injections, and other site immunizations to improve the antibody titer.
4. What is the principle of neutralizing polyclonal antibodies? What principle does it work?
A: When the antigen is injected into an animal, a series of immune cells bind differently to the antigens, and then the cells stimulated by the antigen produce different antibodies, which enter the body through the blood, called polyclonal antibodies. Multiple antibody molecules in the polyclonal antibodies can bind to different antigenic sites, so the polyclonal antibodies have good binding activity. The immunization program is generally divided into three stages, the first is the first immunization, which stimulates their response and produces antibodies. Then, strengthen the immunity, use the antigen to stimulate again, increase the production of antibodies, and finally, take the blood, separate the serum, and determine the titer of the antibody in the serum. Usually, the body has high affinity neutralizing antibodies 28-42 days after antigen injection. Neutralizing antibodies are ways that prevent receptors and cells on the surface of the pathogen and then enter the cells to prevent pathogens from invading the body. Neutralizing antibodies are generally through passive immunity to complete the body's disease treatment.
5. What are the advantages and disadvantages of the different antibodies? And what are the advantages of neutralizing polyclonal antibodies?
A: Antibodies play a key role in both the field of medicine and cancer therapy, and neutralizing antibodies can combine with the occurrence of the virus to reduce the pathogenesis of the virus, so scientists pay special attention. Antibodies are divided into polyclonal antibodies, hybridoma monoclonal antibodies, and recombinant antibodies expressed by genetic technology, etc., and each antibody has its advantages and disadvantages. With our need for various antibody drugs, how to choose the right antibodies becomes the key to the problem. Neutralizing polyclonal antibodies (PAb) have many antigen-binding sites and can bind to multiple antigens, which is ideal for many applications. Its clonality and binding site diversity can make it more practical in some applications. However, the production of PAbs produced by immunization is limited and cannot be produced continuously. After use, we need to reimmunoprepare antibodies, which limits the wide application of neutralizing polyclonal antibodies. In contrast, monoclonal antibodies (mAbs) produced by hybridomas can be continuously produced and are well stable, but the cost of neutralizing antibody preparation is higher than that of neutralizing poly antibodies. The recombinant monoclonal antibody (rAb) obtained by biological means is similar to the recombinant protein, the whole process is more complicated and the production cost is higher, but the antibody can be modified by genetic means to make it multifunctional.
How to Order?
If you have any questions regarding our services or products, please feel free to contact us by E-mail: [email protected] or Tel: +86-400-621-6806