Service Line:+86-022-82164980
Address:FL-4, Building A5, International Enterprise Community, Tianjin, China
Email:[email protected]
Equipped with advanced antibody engineering technology, KMD Bioscience has built up a mature antibody production platform. The booming development of antibody-based therapeutics sees the increasing demand for humanized antibodies. Immunogenicity can potentially lead to reduced efficacy through rapid clearance or neutralization of the biosimilar, or toxicity due to cross-reaction with proteins in the body, which can have catastrophic consequences for the person. Antibody humanization is used to reduce the immunogenicity of animal monoclonal antibodies and improve their activities in the human immune system. It is a very critical step in therapeutic antibody discovery process.
KMD Bioscience provides quality assurance for humanized monoclonal antibody services, including mice, rats, rabbits, etc. Humanization monoclonal antibody refers to the process of tailoring non-human monoclonals to work within human immune systems in an effort to minimize immunogenicity while increasing therapeutic efficacy by including human antibody sequences into existing non-human frameworks. Humanizing monoclonal antibody development typically begins by selecting an existing non-human monoclonal antibody with desirable binding specificity and affinity to serve as its starting point for humanization.
KMD Bioscience, supported by our antibody engineering platform, can offer efficient and accurate antibody humanization service to the scientific community. We have developed antibody humanization strategies based on our phage display technology, which can maintain the specificity and affinity of antibodies to antigens, while significantly or eliminating the immunogenicity of antibodies in humans. KMD Bioscience provides multiple strategies, such as human framework selection, CDR (Complementarity-determining regions) back mutation library screening, etc. KMD Bioscience is devoted to providing comprehensive technical services, including protein expression, antibody production, antibody purification, affinity analysis, and antibody affinity maturation.
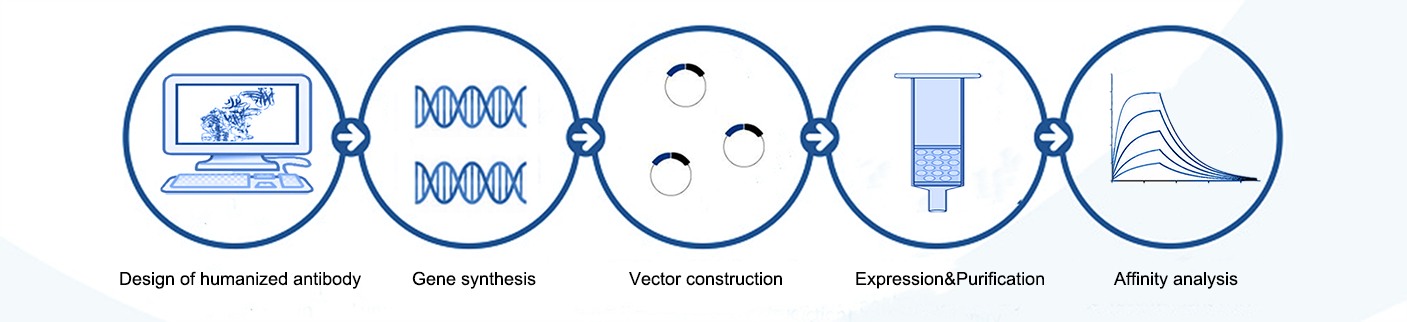
Workflow of Antibody Humanization Service
The client providing the original non-human antibody sequence and relevant information about the target antigen and desired therapeutic properties. Based on this, KMD Bioscience offers expert guidance on the optimal humanization approach. This involves selecting the appropriate human framework, identifying complementarity-determining regions (CDRs), and designing the humanized antibody. Subsequently, we proceed with antibody expression and purification. The humanized antibody then undergoes rigorous in vitro and in vivo testing to evaluate its efficacy and safety.
Antibody Humanization Service Content
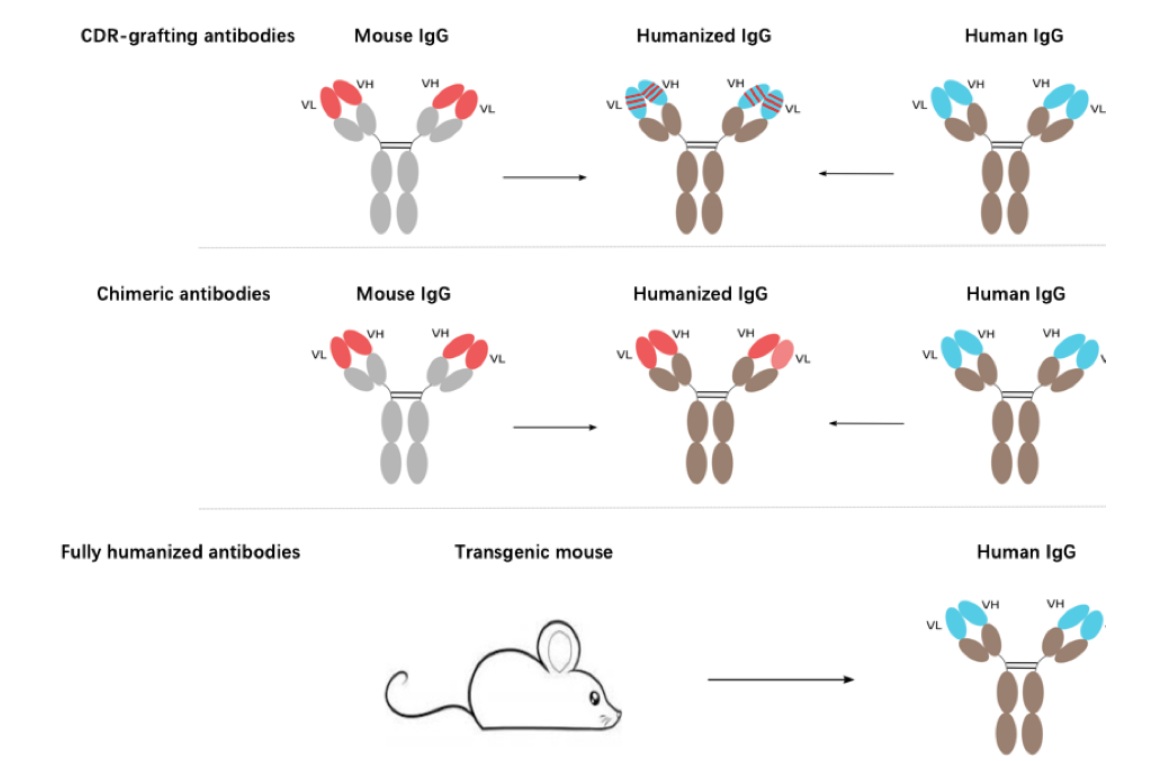
KMD Bioscience can provide three antibody humanization strategies:
CDR-Grafting Antibody: This process involves transferring the antibody CDRs into a proper human antibody framework, while retaining their original affinity and specificity. Such as grafting of the murine CDRs into human frameworks.
Chimeric Antibody: Chimeric antibody is an engineered type of recombinant antibody designed by combining the variable regions from non-human (such as mouse) antibodies with human constant regions in order to create hybrid molecules which maintain specificity and affinity while attenuating immunogenicity and adverse reactions in humans due to human constant regions being added into them. The creation of chimeric antibody (non-human variable regions with human constant regions) can partially alleviate immunogenic response. They were transfected to mammalian cells for recombinant antibody expression using DNA recombination technology.
Fully Humanized Antibody: Production of fully humanized antibodies begins by immunizing mice or rats with antigens that stimulate antibody production in somatic cells (typically B cells) isolated from these animals and extracted. Once this antibody production occurs, gene editing techniques such as homologous recombination or CRISPR-Cas9 may be utilized to replace animal antibodies with humanized versions; modified somatic cells then serve to produce transgenic animals capable of producing fully humanized antibody production.
Antibody Humanization Service Highlights
--Extensive experience in antibody engineering development
--Guaranteed no loss of affinity
--High throughput expression analysis
--Detailed sequence analysis
--Strict quality assurance: cGMP management rules
--A variety of antibody humanization strategies are available
--Various application validation: SPR, ELISA, FACS
--Offer humanization from non-murine origin species, including rabbit antibodies and single-domain camelid nanobodies.
How to Order?
If you have any questions regarding our services or products, please feel free to contact us by E-mail: [email protected] or Tel: +86-400-621-6806