2023-07-24 Hits(479)
A neutralizing antibody (NAb) is a special antibody that specifically binds to and neutralizes bacterial toxins, pathogens (such as viruses) and their products. When pathogenic microorganisms invade the body, corresponding antibodies will be produced, and neutralizing antibodies can bind to antigens on the surface of the virus, and directly prevent the virus from adhering to target cell receptors to prevent the virus from invading cells. Some antibodies will be able to bind to specific proteins and block their function called neutralizing antibodies.
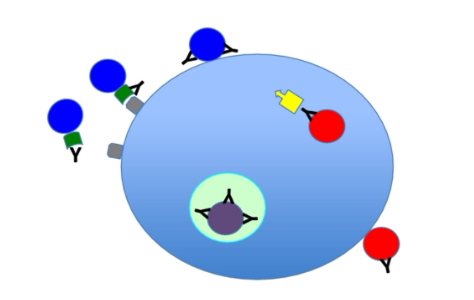
Figure 1: Mechanism of neutralization
NtAb is part of the adaptive immune system's humoral response to viruses, intracellular bacteria, and microbial toxins. They not only bind to viruses but also in a way that stops the infection. NtAb can protect cells from pathogens or infectious particles by neutralizing any of their biological effects. By specifically binding to the surface structure of infectious particles, NtAb prevents them from interacting with host cells, making them more likely to be infected and destroyed. Therefore, NtAbs are sufficient to prevent viral infections.
Neutralizing antibodies can stop pathogens from infecting the body by influencing molecules on the surface of pathogens entering cells in the body. In envelope viruses (heat-sensitive viral cells within the lipid membrane), neutralizing antibodies prevent the virus from attaching to the cell and the virus from entering the cell. In capsular viruses (heat-resistant viral cells without a lipid membrane), neutralizing antibodies can bind to capsid proteins, which are protein shells that surround genetic information within viral cells.
Neutralizing antibodies can also make pathogens and change their structure and shape (called conformational changes) to stop them from entering the cell and replicating within the cell. In bacterial infections, neutralizing antibodies block the harmful effects of toxins. This has been shown to occur in diphtheria medications, although they are no longer recommended to prevent diphtheria infection. Once the pathogen is neutralized by NAb, the pathogen is degraded by white blood cells and the spleen filters the pathogen and then excretes it in urine or feces.
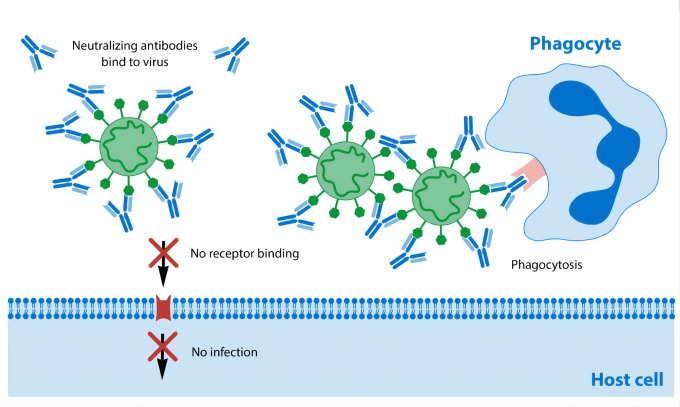
Figure 2: The working principle of neutralizing antibodies
There are currently a variety of methods available for neutralizing antibody production, including hybridoma technology, phage display technology, antibody humanization, etc. Camed Biologics can provide high-quality neutralizing antibody production services. Neutralizing antibody production services provided by Carmed Biologics only accept viral samples. The main experimental process is as follows: antigen production→ animal immunization→ obtaining antiserum →antibody purification → titer detection→ customer delivery serum.
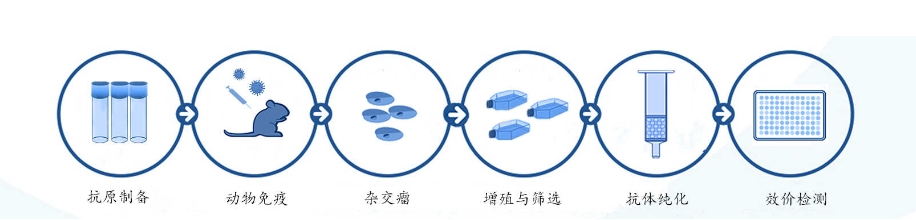
Figure 3: production process of neutralizing antibodies
Currently, the function of NtAbs is studied from two broad perspectives: binding and neutralizing. Antibody binding is often studied by methods such as enzyme-linked immunosorbent assay (ELISA) and flow cytometry (FC). Neutralization is usually assessed by pseudoviral reporter analysis in immortal cells.
ELISA is a common method for analyzing biomolecular interactions. As a highly sensitive, specific, and off-the-shelf technique, it is routinely used in almost every laboratory and can be performed and adapted in a variety of ways. Inhibition of ELISA is a single-point binding analysis method. It can monitor protein-protein interactions in solution compared to the frequently used sandwich ELISA. Inhibition ELISA is used to probe binding sites one at a time to detect binding partners with infinite conformation in solution. Serial dilutions of analytes in a solution can be used quantitatively to assess the antigenicity or binding activity of the solution.
Most biological products function by combining with some form of another part. FC is used to observe and analyze the interaction between fluorescently labeled ligands and their receptors. It is commonly used to characterize the activity of a product by binding to its specific receptors. FC can measure the phenotypic and functional parameters of cells, has been used to diagnose and monitor disease progression, and demonstrates the biological activity of drugs.
When monoclonal antibodies (mAbs) work by blocking ligand binding to cell surface receptors, in vitro binding assays can be used as an alternative efficacy test for therapeutic mAbs.
Due to their security and versatility, fake viruses have become useful virology tools, especially for emerging viruses. For example, the outbreak of the novel human coronavirus SARS-CoV-2 (severe acute respiratory syndrome coronavirus). Due to the high pathogenicity and contagiousness of SARS-CoV-2 and the lack of effective vaccines and therapeutics, live SARS-CoV-2 must be treated under biosafety level 3 conditions, hindering the development of vaccines and therapeutics. Based on the VSV pseudovirus production system, we have developed a pseudovirus-based neutralization detection method. Serum titer determination during pseudoviral neutralization assays is based on endpoint measurements for the evaluation of SARS-CoV-2 in biosafety level 2 facilities and other viral neutralizing antibodies.
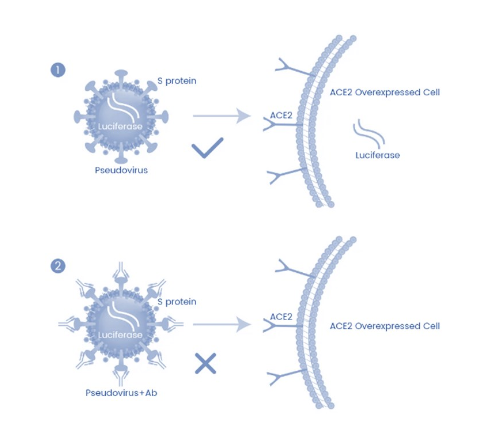
Figure 4: Pseudovirus neutralization detection
Neutralizing antibodies play an important role in the development of antiviral drugs and the production of vaccines. Especially since the outbreak of the new crown virus in 2019, a variety of new crown antibody-neutralizing drugs have been approved for preventive and therapeutic use. KMD Bioscience can provide high specificity and affinity-neutralizing antibody production services, of which bovine-neutralizing antibody production and murine-neutralizing antibody production have been customized for customers over years, KMD Bioscience's mature protein and antibody platforms allow us to provide you with better service.