2024-10-12 Hits(277)
Nanobody
In 1993, a natural antibody lacking a light chain (VL) was discovered in the serum of camelids. Its structure is simple, consisting only of two heavy chains (VH), and it still has antibody activity without Fc. Due to its lack of Fc domain, its volume is small, about 25 kDa, hence it is called nanobody (VHH). Later, this antibody was also found in animals such as alpacas and sharks. nanobodies only contain one heavy chain variable region and two conventional CH2 and CH3 regions. Although they do not have a VL domain, they can still bind to antigens and are highly stable single domain antibodies. They are also the smallest known binding unit with antibody activity. Both nanobodies and traditional antibodies are composed of four relatively conserved framework regions (FRs) and three complementary determining regions (CDRs). However, compared to traditional antibodies, the CDR3 of nanobodies has a circular structural region, which is longer than the typical variable region and has better binding performance. In addition, nanobodies have the characteristic of weak immunogenicity. Humanization of antibodies can further reduce the immunogenicity of recombinant antibodies, thereby reducing the impact on drug treatment efficacy.
Nanobodies can be screened and obtained through phage display technology, and antibody libraries are divided into natural antibody library, immune antibody library, synthetic antibody library, and semi synthetic antibody library. The RNA isolated from the hybridoma was reverse transcribed and used as an amplification template for antibody genes. At the same time, a large antibody gene library containing a large number of nanobodies was created, which could be screened and identified through phage display technology. In addition, ribosome display technology and yeast surface display technology can also obtain high affinity nanobodies. Compared with full-length antibodies, nanobodies are easier to penetrate into tumor tissues and can be applied in targeted cancer therapy drugs. Meanwhile, nanobodies also play an important role in detection reagents and in vitro diagnostics. According to reports, phage display technology can be used to screen for nanobodies targeting Aspergillus flavus, which can then be applied in the field of food safety to detect the content of aflatoxin in agricultural products.
Expression and Purification of Nanobodies
The nanobody expression system includes prokaryotic recombinant antibody expression system and eukaryotic recombinant antibody expression system. Different expression vectors, fusion tags, and host bacteria will produce nanobodies with different activities. Different VHH antibody expression strategies can be developed according to different needs. His tags are widely used in affinity chromatography purification, and VHH fusion of His tags can enable antibodies to specifically bind to metal ions for purification. The operation is simple and suitable for most nanobodies that require efficient purification. The Fc fragment is the constant region of the heavy chain of IgG, which can enhance the stability of antibodies. nanobodies fused with Fc fragments can bind to Fc receptors, trigger immune reactions, and enhance their stability and half-life in vivo. They are suitable for nanobodies that function for a long time in vivo, such as nanobodies used for immunotherapy and drug delivery. Avi tag is a peptide tag that can specifically bind to the biotin ligase BirA to achieve biotinylation of nanobodies. It can then be purified, detected, and delivered using the biotin streptavidin system. The binding of biotin to streptavidin has high specificity and affinity, making it suitable for proteomics, drug screening, disease diagnosis, and more. In addition, fluorescent tags, enzyme cut tags, etc. all have different functions and characteristics. To ensure the expression and correct folding of VHH antibodies, it is necessary to select the VHH antibody expression system according to one's own needs and optimize the expression conditions. The E. coli recombinant antibody expression system is the primary choice for prokaryotic expression. When expressing eukaryotic proteins, the corresponding expression system will be selected based on the characteristics of eukaryotic proteins and different eukaryotic protein expression requirements. Then the nano antibody is expressed and purified. The commonly used antibody purification methods include antibody affinity purification and antibody separation and purification, including Protein A/G purification, ion exchange chromatography, gel filtration, precipitation, and hydrophobic interaction chromatography. According to the characteristics of the antibody and different needs, appropriate purification methods are selected to obtain high-purity antibodies.
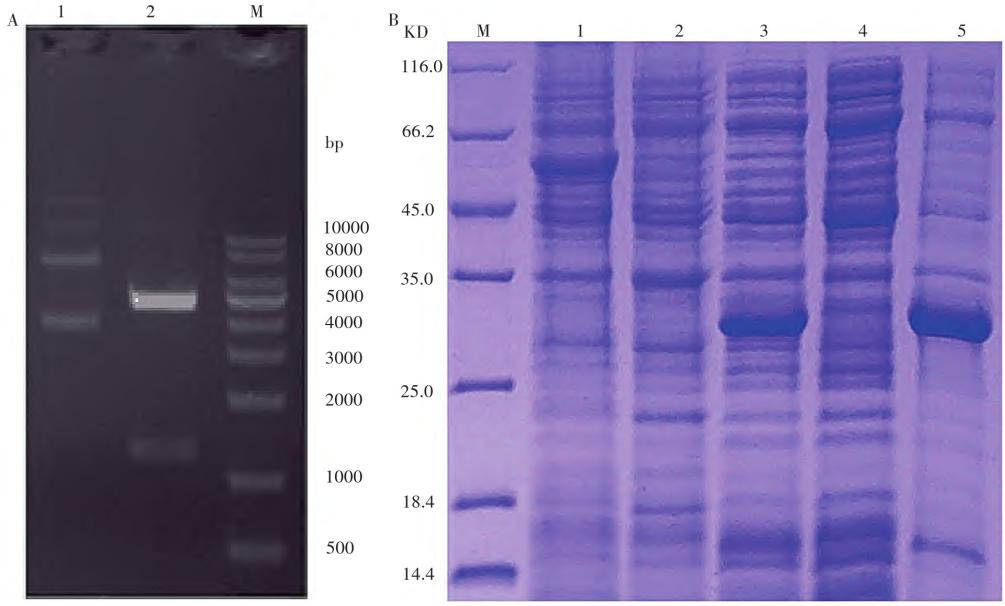
Fig. 1 Expression identification diagram of nanobodies
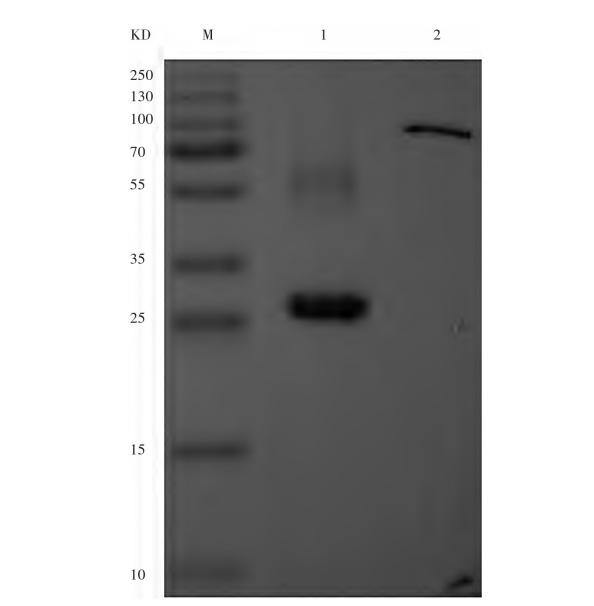
Fig. 2 Western Blot identification image of purified nanobodies
KMD Bioscience has many years of research experience in the field of antibodies and has built a comprehensive antibody customization platform that can provide various types of antibody preparation and development services. In order to ensure that customers can obtain better antibodies, we also provide a series of services such as nanoantibody expression and purification, antibody sequencing, etc. KMD Bioscience has a comprehensive recombinant antibody expression platform, which can use different protein expression systems for nanobody expression and purification according to customer needs. The mammalian expression system can correctly and effectively recognize the synthesis, processing, and secretion signals of eukaryotic proteins, and has a structure and function similar to natural proteins. It can undergo post-translational modifications such as glycosylation, phosphorylation, and acetylation, and the expression and purification process is simple, making it easy to transfect recombinant plasmids. However, mammalian expression systems have the disadvantages of low yield and long preparation cycles. KMD Bioscience has been dedicated to optimizing and researching mammalian expression systems for many years, providing customers with high-yield and high-quality nanobody expression services. We are adept at utilizing HEK293 and CHO cell lines for transient and stable protein expression. We can also use the E. coli prokaryotic expression system to prepare soluble proteins, and optimize the protein expression conditions to avoid protein inclusion body expression and ensure protein activity. The protein expression platform of KMD Bioscience can be used for small-scale and large-scale production of nanobodies to meet the different needs of customers. We can also develop professional antibody humanization strategies for our clients, which can be used to humanized nanobodies. The optimized nanobodies have extremely low immunogenicity, almost equivalent to human antibodies, ensuring their affinity and specificity for antigens. To verify the effectiveness of humanized VHH antibodies, we will characterize the antibodies using ELISA, WB, immunoprecipitation, flow cytometry, immunohistochemistry, endotoxin testing, and other methods to ensure the effectiveness of humanized nanobodies.
Reference
[1] Mahmoudi T, Pourhassan-Moghaddam M, Shirdel B, et al. (Nano)tag-antibody conjugates in rapid tests. J Mater Chem B. 2021;9(27):5414-5438.
[2] Ahmadzadeh M, Farshdari F, Nematollahi L, et al. Anti-HER2 scFv Expression in Escherichia coli SHuffle®T7 Express Cells: Effects on Solubility and Biological Activity. Mol Biotechnol. 2020 , 62(1):18-30.
[3] Bemani P, Mohammadi M, Hakakian A. ScFv Improvement Approaches. Protein Pept Lett. 2018, 25(3):222-229.