2024-07-22 Hits(146)
Fab Antibody
What is a Fab Antibody?
The fragment antigen binding region (Fab region) is the area on an antibody that binds to the antigen. It is a common recombinant antibody fragment, consisting of a constant region on the heavy chain and a variable region on the light chain. It has a larger size, better stability, and affinity, and retains the activity of the intact antibody to bind to the antigen
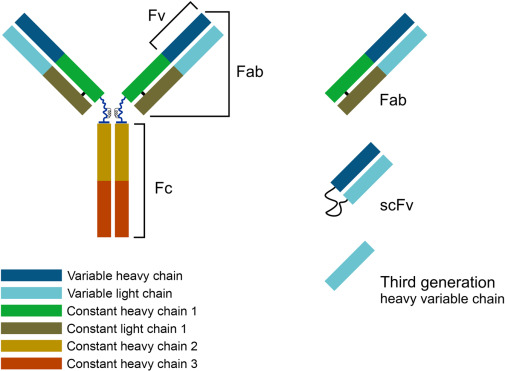
Figure 1 Source: Azadeh Shahidian, Majid Ghassemi, et.al, 2020
Fab Structure
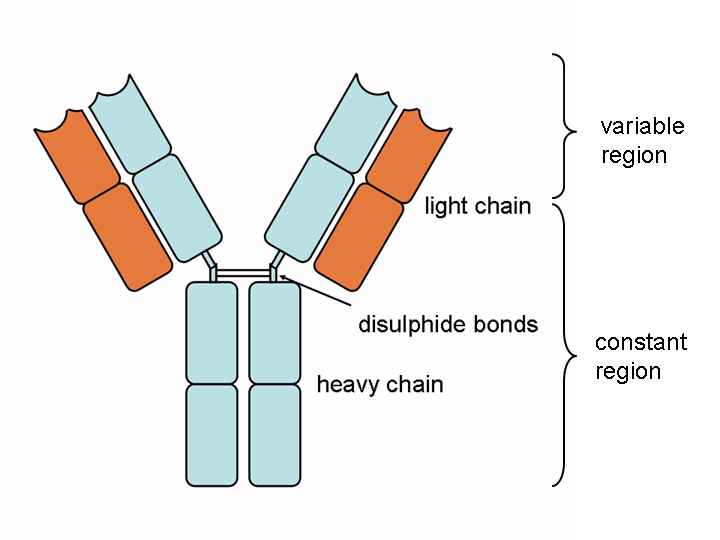
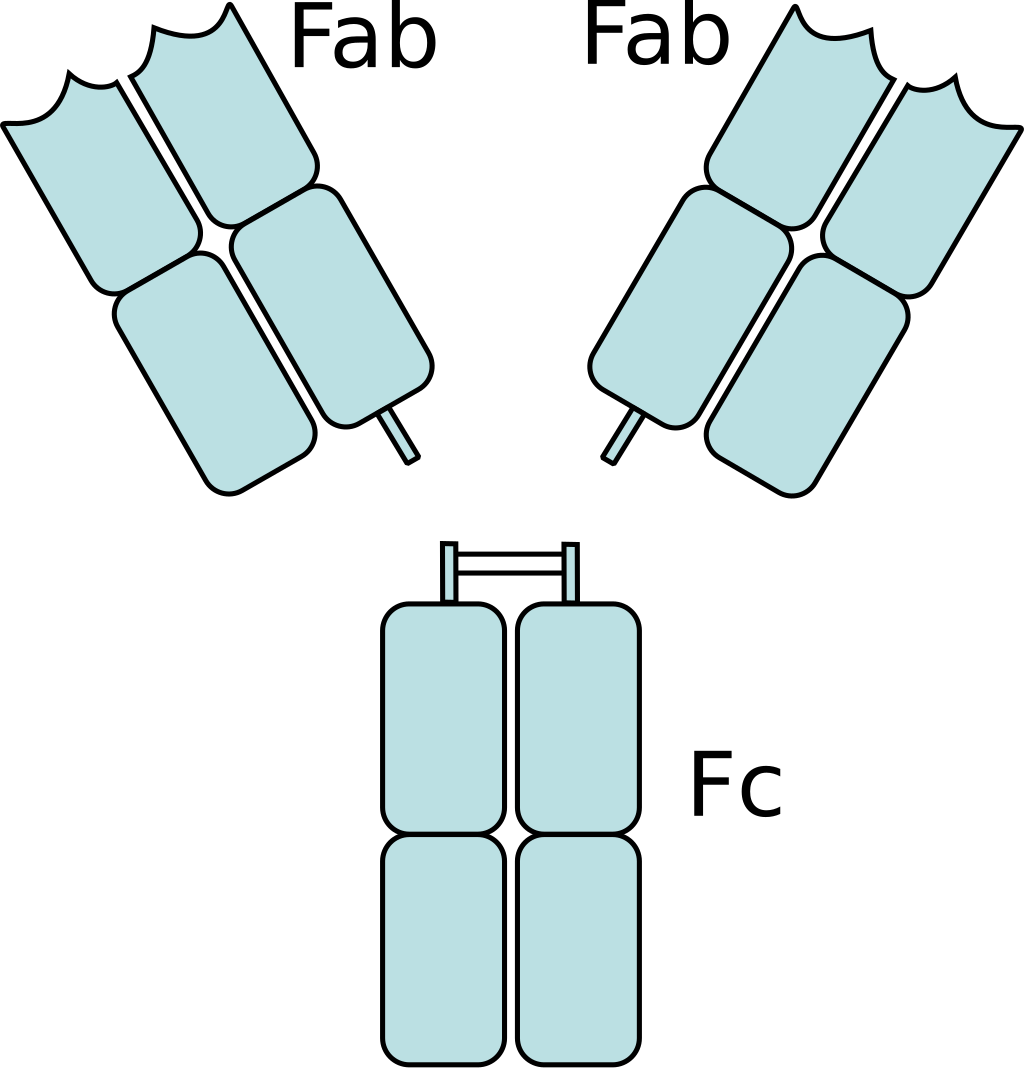
An antibody (also known as immunoglobulin or Ig) is a "Y" - shaped structure composed of two 25 kD light chains and two 50 kD heavy chains connected by disulfide bonds. Fab fragments can be obtained by digesting full-length antibodies with papain, which cleaves immunoglobulin monomers into two Fab fragments and one Fc fragment. If gastric protease cleaves below the hinge region, resulting in F (ab ') 2 fragments and pFc' fragments, while IdeS enzyme (immunoglobulin degrading enzyme of Streptococcus pyogenes) cleaves full-length antibodies in a neutral environment, F (ab ') 2 fragments can be slightly reduced and split into two Fab' fragments. The N-terminus of both chains has a genetically variable domain, which is the source of the enormous antigen binding diversity in the total antibody library. For any given antibody, the C-terminus of each chain is genetically homogeneous and designated as a constant domain (CL or CH). Fab antibodies consist of a variable region (including heavy chain VH and light chain VL, responsible for binding antigens) and a constant region (part of the continuous region: heavy chain constant region 1-CH1 and light chain constant region CL)
 |
 |
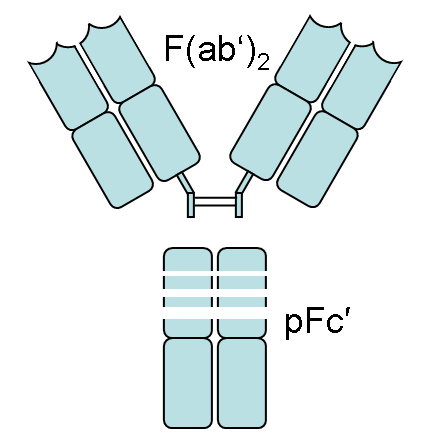 |
| Heavy and light chains, variable and constant regions of an antibody | An antibody digested by papain yields three fragments: two Fab fragments and one Fc fragment | An antibody digested by pepsin yields two fragments: an F(ab')2 fragment and a pFc' fragment |
Figure 2 Source: Wikipedia
Fab Antibody Production
Antibody digestion: The entire antibody is digested to obtain F (ab ') 2 fragments, and then the IdeS enzyme reduces the two F (ab') 2 fragments to split into two Fab fragments, thus obtaining the Fab antibody.
Recombinant technology for producing Fab antibodies: Recombinant Fab fragments are obtained by expressing them in mammalian expression systems (such as CHO or HEK293 cells), prokaryotic expression systems (Escherichia coli), yeast expression systems, insect expression systems (baculovirus), and other expression systems.
Phage display technology: PBMCs are obtained by immunizing animals, RNA is extracted, inverted into cDNA, and the cDNA is amplified in two rounds. The fragments are then transferred into plasmid vectors and expressed in E. coli to construct a Fab antibody library. Three rounds of screening are performed based on suitable targets to obtain specific sequences and produce Fab antibodies.
Fab Antibody Application
Fab fragments can eliminate non-specific binding to cell Fc receptors and serum factors. It has many applications in targeted therapy, immune diagnosis (such as ELISA, and immunohistochemistry), detection (such as quantitative detection of antigens in flow cytometry, detection of specific proteins as primary antibodies in protein immunoblotting, with high specificity and reduced background), structural research, molecular imaging, drug targeted binding, precise drug delivery, etc. Like scFv, Fab fragments have good tumor penetration, rapid blood clearance rate, and low retention time in non-target tissues due to their small size that can penetrate tissues and bind to specific antigens.