2024-09-20 Hits(131)
Sequencing
Since the 1860s, many clinical laboratories have started using mass spectrometry. Among them, liquid chromatography tandem mass spectrometry (LC-MS/MS) technology is mainly used for the quantification of small molecules, such as steroids. And LC-MS/MS technology is the preferred method for quantitatively studying adrenaline in paraganglioma, as well as total and free testosterone in polycystic ovary syndrome. LC-MS/MS technology can analyze multiple substances in the same batch of samples and has high specificity, which helps to identify the results of immunoassays and eliminate misdiagnosis of certain diseases. In addition to being applied in clinical laboratories, LC-MS/MS technology has a wide range of applications in multiple fields. In biomedical research, LC-MS/MS technology can be used for pharmacokinetic studies, proteomic analysis, and can simultaneously detect multiple hormones. For example, in congenital adrenal hyperplasia, LC-MS/MS technology can simultaneously detect a series of hormones such as 17 α - hydroxyprogesterone, 11 deoxycortisol, androstenedione, cortisol, etc. LC-MS/MS technology can be used for protein sequencing, analysis of post-translational modification sites, protein identification, and metabolomics research. LC-MS/MS technology can also detect residues of compounds in food and analyze pollutants in soil, air, and water. With the development of technology, the application prospects of LC-MS/MS technology are still expanding.
The Principle of LC-MS/MS Technology
LC-MS/MS technology, also known as liquid chromatography tandem mass spectrometry, is an extension of liquid chromatography mass spectrometry (LC-MS) by adding a tandem mass spectrometry (MS/MS) step that combines the separation capabilities of liquid chromatography with the analytical capabilities of mass spectrometry. In LC-MS/MS systems, sample components are separated by liquid chromatography and then ionized and analyzed in the first mass spectrometer (MS1). Specific ions are then selected for fragmentation, and the resulting fragment ions are further analyzed in the second mass spectrometer (MS2). When separating sample components, after the sample solution enters the chromatograph, the separation ability of liquid chromatography is utilized to achieve the separation of sample components by utilizing the different interactions and migration rates between different substances and the stationary and mobile phases in the chromatograph. The separated different sample components will be ionized to form charged ions, and the ionized ions will enter the mass spectrometer for analysis. The MS1 mass spectrometer is used to analyze and filter precursor ions, while the MS2 mass spectrometer is used to analyze fragment ions produced after precursor ion fragmentation. By using the spectrum of fragment ions, more structural information about the analyte can be obtained.
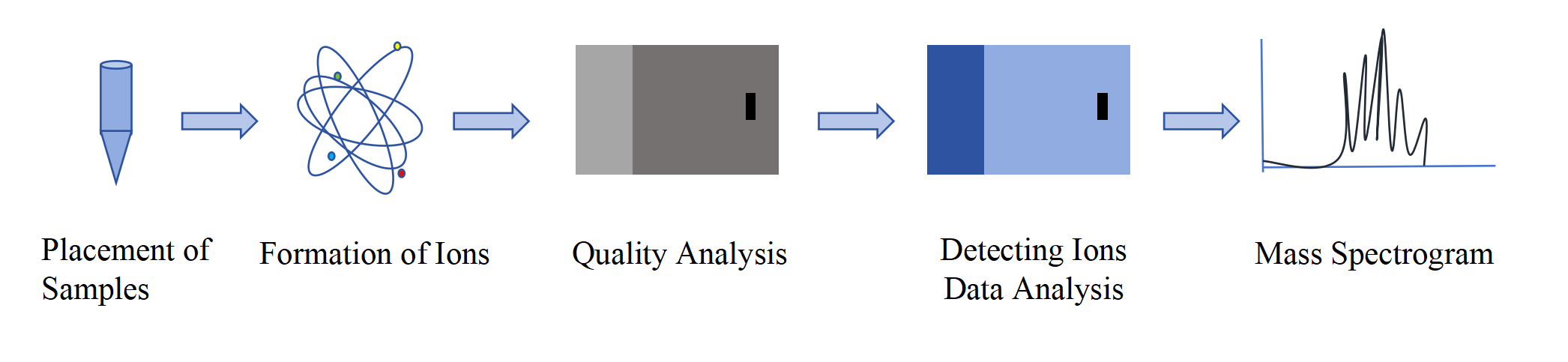
Fig.1 LC-MS/MS flowchart
Sample Requirements for LC-MS/MS Technology
LC-MS/MS technology is suitable for protein identification of various types of samples, including recombinant proteins, bacterial samples, and animal and plant tissue samples. The processing of samples is related to the accuracy of subsequent analysis. Generally, samples containing target proteins suitable for subsequent analysis are obtained from the original samples through steps such as tissue fragmentation, protein extraction, and enzyme digestion. For recombinant protein samples, impurities in the sample should be removed through purification, and the protease should be hydrolyzed into peptide fragments. In addition, salt in the sample should also be removed to reduce salt contamination of the mass spectrometer. For bacterial samples, they need to be first broken down using methods such as ultrasound and high pressure, and then target molecules such as proteins or bacterial metabolites are extracted. For animal and plant tissue samples, the tissue should be ground and crushed first, then the target molecules should be extracted, and impurities should be removed through extraction. When handling samples, it is important to avoid cross contamination and external contamination. To ensure the stability of the sample and avoid degradation or transformation of the target compound during processing, protective agents can be added to protect easily degradable substances. In addition, suitable solvents should be selected based on the solubility of the target compound during the processing.
Mass spectrometry identification and Mass spectrometry coverage
In proteomics research, mass spectrometry identification and mass spectrometry coverage are two different concepts. Mass spectrometry identification refers to the process of using mass spectrometry technology, such as LC-MS/MS, to qualitatively and quantitatively analyze proteins, peptides, or other biomolecules, and obtain information such as molecular weight and structure. Mass spectrometry identification can identify unknown or known molecules and is commonly used to identify unknown proteins in samples. By comparing the obtained data with databases, the type of protein can be identified. In addition, mass spectrometry identification can also analyze the characteristic peptide segments of known proteins to confirm their existence. Mass spectrometry coverage refers to the proportion of protein sequences covered by successfully identified peptides in mass spectrometry analysis, usually expressed as a percentage. It reflects the degree of coverage of protein sequences by mass spectrometry analysis and is also a key indicator for evaluating the quality of mass spectrometry analysis results. Proteins with high mass spectrometry coverage can provide more detailed information on their functions and structures.
Interpretation of LC-MS/MS related data
The final step of LC-MS/MS technology is to screen, proofread, and analyze the data, interpreting useful biological information from a large amount of data to complete protein identification. The data generated by LC-MS/MS mainly includes mass spectra and chromatograms. Among them, the mass spectrum displays the mass to charge ratio information of the substance, while the chromatogram shows the distribution of different compounds over time. The data types include total ion chromatogram (TIC), extracted ion chromatogram (EIC), and tandem mass spectrometry (MS/MS). Firstly, the peak shape of the TIC graph is observed to understand the complexity and distribution range of compounds in the sample. The main and secondary peaks in the graph represent the main components in the sample. According to the mass to charge ratio of the target compound, the corresponding peak in the EIC graph is searched, and the peak height, peak area, and peak retention time are analyzed. When analyzing MS/MS, the mass to charge ratio of the parent ion is first determined, usually the mass to charge ratio corresponding to the main peak in the TIC or EIC diagram. Then, the sub ion spectrum generated after the parent ion fragmentation is observed, and the mass to charge ratio and relative intensity of the sub ions are analyzed. Based on the fragmentation pattern of the daughter ion, combined with the chemical properties of the compound and known fragmentation patterns, the possible structure of the parent ion can be inferred. Finally, the tandem mass spectrum will be compared with known compound databases to confirm the identity of the target compound.
KMD Bioscience can provide various sample preparation and LC-MS/MS detection services for different project requirements. The laboratory uses the Orbitrap Fusion Lumos mass spectrometer. Compared with the previous generation Orbitrap Elite mass spectrometer, Lumos improves the scanning speed of primary and secondary mass spectrometry while ensuring the accuracy of peptide molecular weight. KMD Bioscience has high-precision analytical instruments, experienced operations, and strict quality management to ensure accurate and reliable results.
Reference
[1] Yu S, Zou Y, Ma X, et al. Evolution of LC-MS/MS in clinical laboratories. Clin Chim Acta. 2024;555:117797.
[2] Seger C, Salzmann L. After another decade: LC-MS/MS became routine in clinical diagnostics. Clin Biochem. 2020;82:2-11.
[3] Kobayashi H, Imai K. Recent Progress in FD-LC-MS/MS Proteomics Method. Front Chem. 2021;9:640336.