2024-05-28 Hits(151)
Nanobody Discovery
Background Introduction
Antibodies are biological protein molecules produced by immune B cells stimulated by antigens that can bind specifically to antigens. Due to its ability to bind antigens with high specificity and affinity, antibodies are widely used in academic research, disease diagnosis, and various aspects of medical drugs[1].
A conventional antibody molecule (IgG) is a rather conserved protein molecule consisting of two identical heavy chains and two identical light chains. The light chain of an antibody contains one VL region and one CL region, while the heavy chain has one VH region and three CH regions (CH1, CH2, and CH3).
VH region and VL region together constitute the smallest unit of antigen recognition by traditional antibodies, and the sequence difference of antibody variable region determines that antibodies can specifically recognize different antigens[2]. On the other hand, the CL region and CH region are relatively conserved and known as the constant region of antibodies, in which the CH2 and CH3 regions of the CH region play an important role in recruiting immune cells for ADCC and CDC functions.
Heavy Chain antibodies are special antibodies naturally occurring in camels and cartilaginous fish that are composed of only two heavy chain antibodies in addition to traditional antibodies and contain only one Variable Domain of Heavy Chain Antibody (VHH) and two conventional CH2 and CH3 regions, with the absence of CH1 region. Heavy chain antibodies bind to antigens via a variable region (VHH) on the heavy chain that can be stably present alone in vitro and are called camel single domain nanobody (SdAb) or nanobody. The nanobody crystal is 2.5nm wide and 4nm long, and the molecular weight is only 1/10 (about 15kD) of the traditional complete antibody, but it still has complete antigen recognition ability, and the VHH sequence is generally obtained by phage screening.
Thanks to the tiny structure, complete antigen recognition ability, and phage screening technology, the complete VHH sequence can be obtained, and the nanobody can be mass-produced by recombinant expression in vitro, effectively avoiding the problem of batch-to-batch difference of traditional antibodies.
Compared with traditional antibodies, nanobodies have small molecular weight and simple structure. Due to the advantages of small molecular weight, nanobodies have many features, which makes nanobody show great potential in new drug discovery[3]: They have stronger specificity in binding to targets, and can bind to sites that traditional antibodies cannot bind to; Higher tissue penetration; Higher stability such as high-temperature resistance; Suitable for industrial large-scale production; Easier to transform and optimize; Easier to humanize.
Because of these characteristics of nanobodies, more and more research institutions and drug manufacturers pay attention to and try to use nanobodies in different scenarios. The development of nanobody is different from the traditional monoclonal antibody production method by hybridoma, which generally selects candidate nanobody by immunizing alpaca, constructing a phage library and displaying phage, and then carries out the verification experiment of binding to antigen after expression and purification of nanobody.
.png)
Figure 1: The difference between conventional antibodies and nanobodies
Introduction to Immunogen Types
At present, the acquisition of single-domain antibodies is generally achieved by immunizing alpaca and maturing the antibodies of the immune system itself in alpaca. B lymphocytes are separated, RNA is extracted, cDNA is obtained by reverse transcription, cDNA is used as the substrate for PCR amplification to obtain diversified nanoparticle antibody gene fragments, and then the diversified nanoparticle antibody gene fragments are connected to phage granules to construct phage library. Then, the most suitable antibody was selected from the alpaca antibody library by phage display screening technology and the nanobody was verified. The whole process mainly includes alpaca immunization, phage library construction, antibody screening, expression purification, and verification.
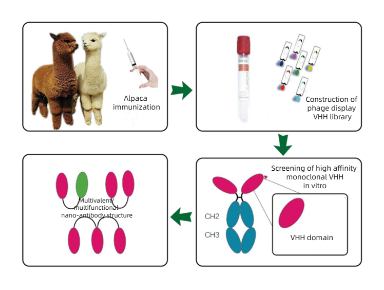
Figure 2: The process of nanobody production
The immunogens used to immunize alpacas:
(1) Protein immunogen: Protein immunogen is generally composed of whole proteins or recombinant protein fragments, which can well simulate the epitope of the antigen under natural conditions, stimulate a strong immune response, and thus induce the production of nano antibodies with high affinity and specificity.
(2) Small molecule immunogen: Small molecule usually refers to the molecular weight of less than 1 kDa compound. Due to its low molecular weight, it is not sufficient on its own to trigger a response from the immune system, so it needs to be coupled with a larger carrier protein to enhance its immunogenicity.
(3) Polypeptide immunogens: Polypeptide immunogens are composed of short-chain amino acids, can be linear or conformationally qualified, and are usually used to simulate local epitopes of protein antigens, which need to be properly designed and modified to improve their stability and immunogenicity.
(4) Virus immunogens: Viral immunogens are specific proteins or virus particles extracted from viruses that induce an immune response from the host. These immunogens are usually inactivated, unable to cause disease, but sufficient for the host's immune system to produce a defense against the virus.
(5) DNA immunogens: This type of immunogen is usually prepared by synthesizing the coding DNA sequence of the target protein. The advantage of DNA immunogen is that it can precisely control the coding sequence of proteins, enabling the production of specific antibodies. In addition, DNA immunogen preparation is relatively simple and cost-effective.
(6) RNA immunogens: RNA immunogens are very useful for studying specific RNA subtypes and post-transcriptional modifications. However, it is difficult to maintain its stability and complex to operate.
(7) Cell line type Immunogens: This is a method that uses the cell line as the immunogen, usually through the expression of the target protein by the cell line to produce antibodies.
Immunization Technology
(1) The selection of alpaca and the immune antigen are the key to the success of immunization. Choose a healthy and strong, good mental state, moderate size of the alpaca is blank alpaca. The purity of the immune antigen and its correct conformation are crucial to the screening of suitable antibodies after immunizing alpacas for subsequent use, and the purity of the protein antigen is generally not less than 90%.
(2) lymphocyte separation: Timely cell separation can effectively prevent hemolysis after blood collection to achieve the best separation effect.
(3) The choice of the immune cycle can affect the immune effect, according to experience, 1-2 weeks of immunization interval can make alpacas have a good immune response to most antigens.
References
[1] Cortez-Retamozo V. Efficient Cancer Therapy with a Nanobody-Based Conjugate[J]. Cancer Research, 2004, 64(8):2853-2857.DOI:10.1158/0008-5472.CAN-03-3935.
[2] Meyer T D, Muyldermans S, Depicker A . Nanobody-based products as research and diagnostic tools[J]. Trends in Biotechnology, 2014, 32(5).DOI:10.1016/j.tibtech.2014.03.001.
[3] Steyaert J, Kobilka B K. Nanobody stabilization of G protein-coupled receptor conformational states.[J]. Current Opinion in Structural Biology, 2011, 21(4):567-572.DOI:10.1016/j.sbi.2011.06.011.