2025-02-28 Hits(48)
Monoclonal Antibody
A monoclonal antibody (mAb) is a highly uniform antibody produced from a single B cell clone that targets only a specific epitope by hybridoma technology. The types include murine monoclonal antibodies, chimeric monoclonal antibodies, human monoclonal antibodies, and fully human monoclonal antibodies. The techniques of monoclonal antibody preparation include the hybridoma technique, phage display technique, and single B cell antibody production technique. Among them, hybridoma technology is mature and more used, and the research cost is low.
Monoclonal antibodies are widely used in the prevention, diagnosis, and treatment of diseases, especially in the clinical treatment of tumors. The first therapeutic monoclonal antibody (to prevent rejection of kidney transplants) was approved in 1986, and then several monoclonal antibody products have been approved for the treatment of various diseases (asthma, rheumatoid arthritis, etc.).
Principle
B cells from immunized mice were fused with myeloma cells to form hybridoma cells. Hybridoma cells were screened to obtain specific antibody secretion functions, and then a monoclonal antibody was obtained (Fig.1). The homologous antibody produced by monoclonal hybridoma cells obtained by clonal fusion, screening, and cloning of single epitope-specific B cells is called monoclonal antibody.
Traditional hybridoma technology is unable to selectively fuse B cells and myeloma cells, to overcome this problem, a new hybridoma technology has been developed. The new technique is based on the preselection of B lymphocytes with target antigens of immunoglobulin receptors, and the selective fusion of B-cell-myeloma cell complexes with electrical impulses, and is known as B-cell targeting, multitargeting, and stereospecific targeting. It can meet a variety of needs, not only producing a single monoclonal antibody but also producing monoclonal antibodies and selectively producing stereospecific monoclonal antibodies. At present, the technology can be used to produce human monoclonal antibodies for clinical purposes.
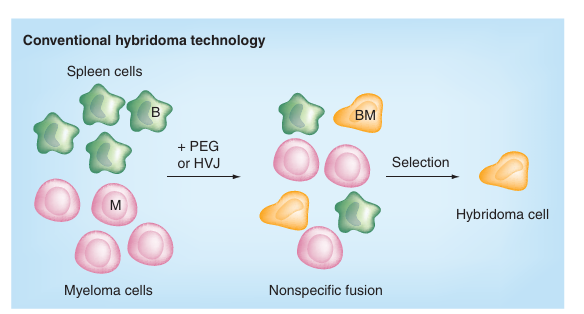
Fig 1 Hybridoma technology principle
(Reference source: Sci-Hub | Hybridoma technologies for antibody production. Immunotherapy, 3(3), 371–380 | 10.2217/imt.11.4)
Mouse Monoclonal Antibody Production Process
The traditional hybridoma technique was introduced by taking the process of monoclonal antibody production in mice.
(1) Mouse immunity
Female BALA/c mice aged 6-8 weeks are generally selected to be immunized with specific antigens to stimulate immune cells to produce specific antibodies. The specific immunization regimen is determined according to the needs of the experiment. For soluble antigens, the immunization should be mixed with adjuvants in equal proportions (complete and incomplete Freund’s adjuvants).
(2) Isolation of B lymphocytes
After the end of the immune cycle, the mice were killed, the spleen of the mice was dissected under sterile conditions, the cell suspension was prepared by extrusion and grinding, and the needle B lymphocytes were isolated.
(3) Fusion cell production
The splenic cells were fused with myeloma in a certain proportion and cultured with the fusion promoter polyethylene glycol to form hybridoma cells.
(4) Hybridoma cells were screened
Hybridoma cells were screened using a specific HAT medium and then screened for hybridoma cells producing specific antibodies (limited dilution, ELISA).
The screening principle of the HAT selective medium is based on the nucleotide synthesis pathway. A (aminopterin) can block the total synthesis pathway of nucleotides, and H (hypoxanthine) and T (thymine nucleoside) can synthesize nucleotides by a remedial synthesis pathway. Unfused myeloma cells cannot use the remedial pathway to synthesize DNA due to a lack of hypoxanthine-guanine-phosphoribosyl transferase; Unfused lymphocytes do not survive because they do not survive in vitro for long periods, despite the presence of hypoxanthine-guanine-phosphoribosyl transferase; The fused hybridoma cells could survive and proliferate in a HAT medium that transferase was obtained from spleen cells, which had the property of infinite proliferation of myeloma cells.
(5) Monoclonal antibody culture
Single hybridoma cells were isolated for monoclonal antibody culture to obtain a monoclonal antibody cell line. The methods of antibody production include in vivo induction and in vitro culture.
(6) Monoclonal antibody extraction
After harvesting growing monoclonal antibody cell lines, antibodies were extracted and sequenced by a specific method and sequenced.
(7) Antibody purification
The monoclonal antibody is purified by separating the antibody from other proteins.
Large Quantities of Production
The methods of Monoclonal antibody production include in vivo induction and in vitro culture. The in vivo induction method is to inject the tumor cells into the abdominal cavity of mice and obtain monoclonal antibodies through ascites. The in vitro culture method is to culture hybridoma cells in a culture bottle, collect culture liquid, concentrate, and purify. Both of the above methods require stable hybridoma cells, and hybridoma sequencing can avoid the risk of loss and ensure the accuracy of the sequence, antibody sequencing can also be performed.
KMD Bioscience provides monoclonal antibody production services to customers, including mouse monoclonal antibody production, mouse monoclonal antibody customization, and antibody production services. In particular, mouse monoclonal antibody customization services include hybridoma antibody technology, phage antibody display library technology, and single B cell antibody technology. In addition, KMD Bioscience also provides antibody purification, antibody sequencing, and other services.
References:
[1] Kohler G, Milstein C. Continuous cultures of fused cells secreting antibody of predefined specificity [J]. Nature. 1975, 256(5517): 495-497.
[2] Tomita M, Tsumoto K. Hybridoma technologies for antibody production [J]. Immunotherapy. 2011, 3(3): 371-380.
[3] Stills HF Jr. Adjuvants and Antibody Production: Dispelling the Myths Associated with Freund’s Complete and Other Adjuvants [J]. ILAR J. 2005, 46(3): 280–293.