2024-09-12 Hits(142)
Recombinant Antibodies
Since the approval of monoclonal antibodies by the US Food and Drug Administration in 1986, an increasing number of antibodies have been used for disease treatment. The variable domain, light chain variable region (VL), and heavy chain variable region (VH) form monoclonal antibodies, also known as recombinant antibodies, which recognize target antigens through their variable fragments. In the 1980s, BIrd et al. designed single-chain antibodies (scFv) as a genetic fusion of VL and VH, which links VL and VH through short flexible peptides. scFv is the smallest binding unit with antibody activity. Due to its lack of Fc domain, its volume is minute, about 25 kDa. Compared with full-length antibodies, it is easier to penetrate tumor tissue and can be applied in targeted cancer treatment drugs. At the same time, it also plays an important role in detection reagents. In addition, scFv has the characteristic of weak immunogenicity, and the humanization technology of scFv can further reduce the immunogenicity of recombinant antibodies, thereby reducing the impact on drug treatment efficacy.
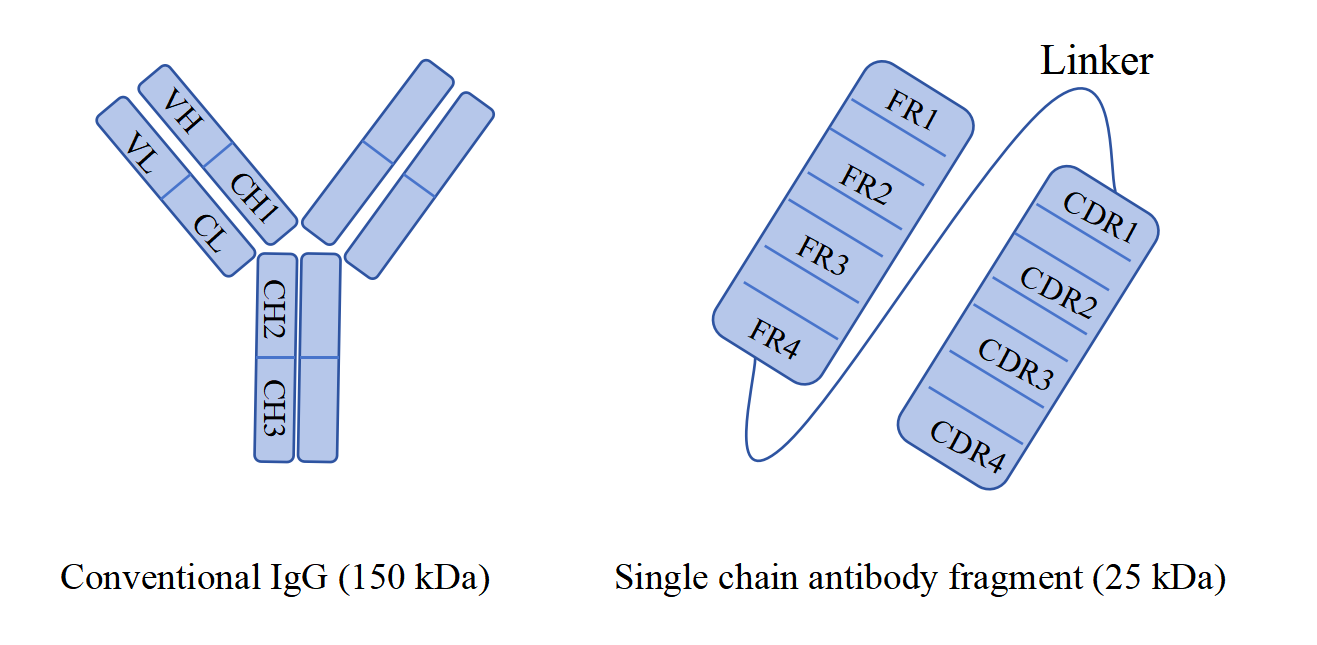
Fig. 1 Structures of conventional antibody and single chain antibody fragment
Single Chain Antibody Display Technology
The scFv antibody phage display platform can screen and obtain scFv. The antibody library is divided into natural antibody library, immune antibody library, synthetic antibody library, and semi synthetic antibody library. RNA isolated from hybridoma was reverse transcribed as an amplification template for antibody genes, and a large antibody gene library containing a large amount of scFv was created. Subsequently, phage display technology was used for screening and identification. The phage display platform is easy to operate and has the advantages of high throughput and low cost. It can also avoid the effects of high temperature, pH, and protease. However, due to the different binding characteristics of antibodies in different cells, phage display technology has certain limitations in library capacity, and not all genes can be expressed on phage display platforms. At present, phage display technology is mainly applied in research related to biomedicine, especially in antibody drug development and target protein screening. In addition, ribosome display technology and yeast surface display technology can also obtain high affinity single chain antibodies.
Single Chain Antibody Expression System
The main expression systems of scFv include prokaryotic expression system and eukaryotic expression system. Among them, due to the fast growth rate and low cost of Escherichia coli, prokaryotic expression system is the most commonly used expression system in recombinant antibody customization and recombinant antibody expression service. In prokaryotic expression systems, different expression vectors, fusion tags, and host bacteria all produce different activities of scFv.
Firstly, in terms of expression vectors, PET series has become the preferred choice for protein expression in Escherichia coli and is currently a commonly used vector for expressing scFv. In addition, bacteriophage vectors such as pIT2 can also be used as an option. For the convenience of subsequent purification and functional verification, fusion tags are often added, such as His tags, Fc tags, SUMO tags, etc. His tags can be used for affinity chromatography purification of scFv and have become the most commonly used fusion tags. The K-12 and B series of Escherichia coli strains are commonly used host bacteria in production at present, while BL (DE3) strains are more common in the laboratory. However, due to certain defects in the expression of codons in Escherichia coli, some codons in scFv may not be expressed. KMD Bioscience obtained a dual expression plasmid by modifying the pET vector, which contains dual MCS sites, dual T7 promoters, dual lac operons, and dual ribosome binding sites. During the recombinant antibody customization and recombinant antibody expression service, affinity tag fusion expression was used, including short peptide tags and long peptides co-expressed in the form of fusion proteins. Rosetta 2, as a host for prokaryotic expression, is a good choice as it can supplement the tRNA corresponding to the seven rare codons lacking in Escherichia coli.
ScFv is Expressed Through the Prokaryotic Expression System
After obtaining the antibody gene library and screening through phage display technology, the scFv obtained can express antibody proteins through various expression systems. The use of prokaryotic expression has the advantages of fast expression speed, low cost, and simple method. However, some small molecule proteins may require complex protein renaturation treatments in the later stage due to their protein expression in the form of inclusion bodies, such as Leptin, EGF, etc. A high-quality solution and recombinant antibody customization and recombinant antibody expression service are necessary for achieving high levels of solubility and high-yield protein expression in prokaryotic expression systems.
.png)
Fig. 2 Expression and purification of recombinant scFv antibody
KMD Bioscience can provide recombinant antibody expression services to complete the customization of recombinant antibodies. We have multiple prokaryotic expression systems, such as BL21, Nissle 1917, Rosetta, etc. And KMD Bioscience has mature technology and rich experience in handling inclusion body refolding, and can also customize prokaryotic expression schemes according to customer’s needs. Our service advantage lies in the ability to perform codon optimization, which includes rare codon analysis, protein hydrophilicity analysis, and predictive analysis of transmembrane domains. In addition, we can provide multiple fusion labels, multiple expression systems, and a complete GMP document support system.
Reference
[1] Boucher LE, Prinslow EG, Feldkamp M, et al. "Stapling" scFv for multispecific biotherapeutics of superior properties. MAbs. 2023 , 15(1):2195517.
[2] Ahmadzadeh M, Farshdari F, Nematollahi L, et al. Anti-HER2 scFv Expression in Escherichia coli SHuffle®T7 Express Cells: Effects on Solubility and Biological Activity. Mol Biotechnol. 2020 , 62(1):18-30.
[3] Bemani P, Mohammadi M, Hakakian A. ScFv Improvement Approaches. Protein Pept Lett. 2018, 25(3):222-229.