2025-04-15 Hits(135)
Phage Display
Introduction
Phage display is a powerful molecular biology technique that allows the presentation of peptides or proteins on the surface of bacteriophages. This system enables the selection of high-affinity ligands for various targets, making it invaluable in antibody engineering, drug discovery, and protein-protein interaction studies.
Phage display technology, first developed by George P. Smith in 1985, involves the fusion of foreign DNA sequences to phage coat protein genes, resulting in the display of encoded peptides or proteins on the phage surface. This system allows for the rapid screening of large libraries (up to 10^11 variants) through biopanning, where phages binding to a target are enriched and amplified. Phage display is a versatile tool with applications spanning therapeutics, diagnostics, and basic research. While M13 dominates for antibody engineering, T4, T7, and λ phages offer advantages for large protein display and vaccine development. Future advancements may integrate deep learning for library design and cell-free systems for improved folding. This technology continues to evolve, driving innovations in precision medicine and biopharmaceuticals.
Two major classes of phages are used in phage display system:
Filamentous phages (e.g., M13 phage display):Non-lytic, suitable for continuous display.
Lytic phages (e.g., T4, T7, λ): Explode host cells, useful for large protein display.
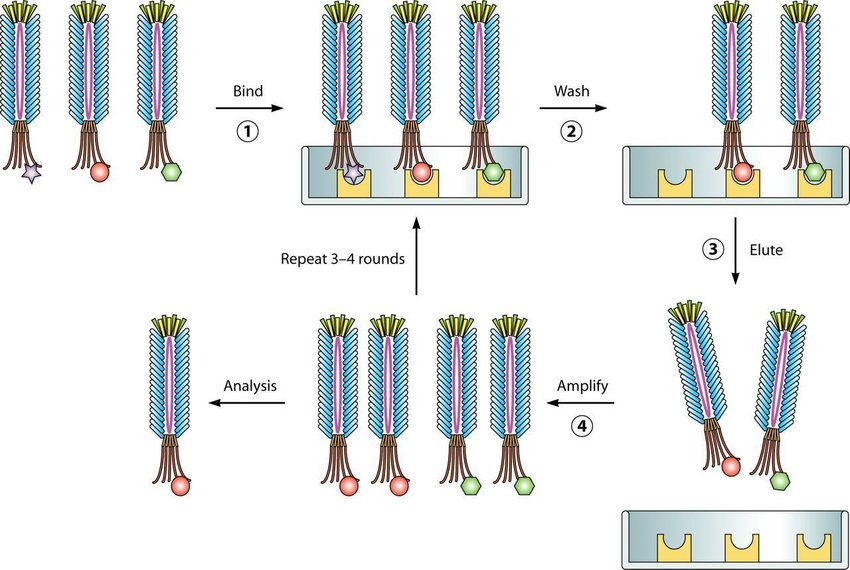
Figure 1:The principle of the phage display system[2].
Types of Phages in Phage Display
(1) Filamentous Phage: M13
① Structure & Lifecycle:
M13 is a single-stranded DNA (ssDNA) phage with a flexible, rod-shaped capsid (~900 nm long). Infects E. coli via the F-pilus (F+ strains like ER2738). Does not lyse the host, instead, it replicates and secretes progeny virions continuously.
② Display Mechanism:
Foreign peptides/proteins are fused to coat proteins:
pIII (minor coat protein, 3-5 copies) – Used for high-affinity display (e.g., antibodies).
pVIII (major coat protein, ~2700 copies) – Suitable for short peptides (≤8 aa).
③ Advantages:
High library diversity (10^9–10^11 clones).
Compatible with affinity maturation (improving binding strength).
Stable virions, easy purification.
④ Limitations:
Limited protein size (large proteins may disrupt phage assembly).
Requires E. coli strains.
(2) Lytic Phages: T4, T7, and λ
Unlike M13, lytic phages replicate rapidly and burst host cells, making them suitable for different applications.
① T4 Phage Display
Structure: Large, double-stranded DNA (dsDNA) phage with an icosahedral head and tail fibers.
Display Sites: SOC (small outer capsid) & HOC (highly antigenic outer capsid) . Can display large proteins (e.g., full-length antibodies).
Advantages: High stability (harsh conditions). Suitable for vaccine development (strong immune response).
② T7 Phage Display
Structure: dsDNA phage with a short lifecycle (~1 hour).
Display Sites: Capsid protein (10B) – Allows multivalent display.
Advantages: Rapid amplification (useful for high-throughput screening). Can display proteins >50 kDa.
③ λ Phage Display
Structure: dsDNA phage with a temperate lifecycle (can lysogenize).
Display Sites: D (decoration) protein & gpV (tail protein).
Advantages: High valency (~400 copies per phage). Useful for cell-targeting studies.
Differences Between Filamentous Phage (M13) and Lytic Phages (T4/T7/λ)
| Project | M13 Phage | T4/T7/λ Phage |
| Genome and Structure | A flexible, filamentous phage with a single-stranded DNA (ssDNA) genome. Its capsid consists of major coat protein pVIII (~2700 copies) and minor coat protein pIII (3-5 copies), making it ideal for displaying small peptides or antibody fragments | These are lytic phages with double-stranded DNA (dsDNA) genomes and icosahedral capsids. T4 uses SOC/HOC proteins for display, T7 uses the 10B capsid protein, and λ phage utilizes the D protein or tail protein gpV. Their rigid structures allow the display of larger proteins (e.g., full-length antibodies). |
| Lifecycle and Host Interaction | Non-lytic, it infects F⁺ E. coli via the F-pilus and continuously secretes progeny virions without killing the host. This makes it suitable for long-term library amplification. | Lytic phages rapidly replicate and burst host cells. T7 has an extremely short lifecycle (~1 hour), enabling high-throughput screening, while T4 and λ can package large DNA inserts but require fresh host cells for each round of propagation. |
| Display Capacity | Best for small-to-medium proteins (≤50 kDa when using pIII). The pVIII protein allows high-density peptide display but is limited to short sequences (≤8 aa). | Capable of displaying large proteins (>100 kDa). T4’s SOC/HOC system is robust for vaccine antigens, T7’s 10B protein supports multivalent display, and λ’s D protein enables high-copy-number presentation (~400 copies/phage). |
| Applications | Dominates antibody engineering (scFv, Fab libraries) and peptide screening due to high library diversity (10^9–10¹^11 clones). |
T4 and T7 phage: preferred for vaccines (strong immune response) and large protein display (e.g., virus-like particles). T7’s speed is ideal for rapid selection. λ phage: Used for studying multivalent interactions (e.g., receptor clustering) but less common than M13/T7. |
M13 excels in antibody and peptide display, while T4/T7/λ are better for large proteins, vaccines, and rapid screening. The choice depends on the target size, desired immune response, and experimental throughput.
Applications of Phage Display
(1) Antibody Engineering
Humanized antibodies (e.g., Adalimumab, derived from phage libraries).
Nanobodies (single-domain antibodies from camelid VHH libraries).
(2) Drug Discovery
Peptide-based inhibitors (e.g., bivalent thrombin inhibitors).
Targeted therapeutics (e.g., cancer-homing peptides).
(3) Epitope Mapping & Protein Interaction Studies
Identifying binding sites (B-cell epitopes for vaccine design).
Studying protein-protein interactions (e.g., receptor-ligand pairs).
(4) Diagnostics & Biosensors
Phage-based ELISA for pathogen detection.
Tumor imaging (phages displaying tumor-targeting peptides).
FAQs about Phage Display
Q1: Why is M13 the most commonly used phage?
A: M13 is preferred due to its high library diversity, non-lytic nature, and ease of purification. It is ideal for antibody fragment (scFv, Fab) display.
Q2: Can phage display be used for non-protein molecules?
A: Yes, DNA/RNA aptamers can be displayed, but peptide/protein display is more common.
Q3: How is phage display different from yeast display?
A: Phage display is faster and cheaper but limited by E. coli expression. Yeast display allows eukaryotic post-translational modifications (e.g., glycosylation).
Q4: What are the limitations of phage display?
A: Bias in panning (some clones dominate). E. coli folding limitations (no mammalian PTMs).
Q5: Can lytic phages replace M13?
A: For large proteins (>50 kDa) or vaccine applications, T4/T7/λ are better. But M13 remains dominant for peptide/antibody libraries.
References
[1] Hawkins R E , Hoogenboom H R , Winter G ,et al.Making Antibodies by Phage Display Technology[J].Annual Review of Immunology, 1994, 12(1):433.DOI:10.1146/annurev.iy.12.040194.002245.
[2] Dammers C, Yolcu D, Kukuk L, Willbold D, Pickhardt M, Mandelkow E, Horn AH, Sticht H, Malhis MN, Will N, Schuster J, Funke SA. Selection and Characterization of Tau Binding ᴅ-Enantiomeric Peptides with Potential for Therapy of Alzheimer Disease. PLoS One. 2016 Dec 22;11(12):e0167432. doi: 10.1371/journal.pone.0167432. PMID: 28006031; PMCID: PMC5179029.
[3] Hoogenboom H R , Chames P .Natural and designer binding sites made by phage display technology[J].Immunology Today, 2000, 21(8):371-378.DOI:10.1016/S0167-5699(00)01667-4.