Service Line:+86-022-82164980
Address:FL-4, Building A5, International Enterprise Community, Tianjin, China
Email:[email protected]
Proteins, biomolecules, or macromolecules, perform various functions in organisms. Almost all of the cellular processes require that proteins specifically recognize a multitude of different interaction partners. They can perform their roles by interacting with other molecules, including DNA, RNA, proteins, and small molecules. Protein interaction assays refer to intentional physical contacts established between two or more proteins due to biochemical events and/or electrostatic forces.
Protein-protein interactions play a crucial role in cellular functions and biological processes in all organisms. The identification of protein interactions can lead to a better understanding of infection mechanisms. Several physiochemical experimental techniques have been applied to identify PPIs.
KMD Bioscience’s protein interaction assay services take advantage of advanced technology and proven expertise to help customers meet their scientific research project needs. KMD Bioscience protein-protein interactions team has rich experience in the field and can provide various advanced and reliable protein interaction assays against the protein-protein interactions of interest in vivo or in vitro. Services we are offering include but are not limited to yeast two-hybrid assay, western blot, pull-down assay, SPR binding assay, BLI assay, Co-immunoprecipitation, and chromatin immunoprecipitation assay (chip assay).
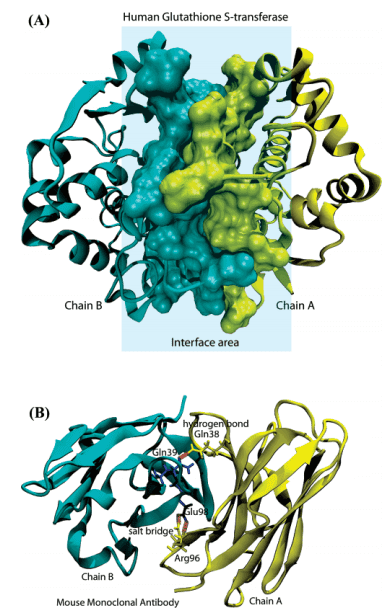
Fig 1: Illustration of protein-protein interfaces
Protein-protein Interactions Assays Service
1. Yeast two-hybrid Assay Service
The yeast two-hybrid system (Y2H) is the most popular method to identify binary PPIs in vivo. A protein of interest 'B' is expressed in yeast as a fusion to a Gal4p DNA-binding domain (DBD, "bait"; circles denote expression plasmids). Another protein or library of proteins of interest 'ORF' is fused to the Gal4p transcriptional activation domain (AD, "prey"). The two yeast strains are mated to combine the two plasmids expressing bait and prey fusion proteins in the same cell (diploid). If proteins 'B' and 'ORF' interact in the resulting diploid cells, they reconstitute a transcription factor that activates a reporter gene (HIS3) and therefore allows the cell to grow on selective synthetic media (media lacking histidine).
Advantages of Yeast Two-Hybrid Assay:
(1) High efficiency and reliability.
(2) The yeast two-hybrid system became one of the most popular technologies for the detection of protein-protein interactions because it is fairly simple, rapid, and inexpensive (avoids the costly protein purification and antibody development needed in the traditional biochemical methods).
(3) No previous knowledge about the interacting proteins is necessary for a screen to be performed.
(4) The system can be scaled up to high-throughput usage.
Limitations of Yeast Two-Hybrid Assay:
1) There are high rates of false positives and false negatives in Y2H screens.
2) Interaction must occur in the nucleus for the reporter gene to be activated.
3) Tagged proteins may not fold correctly and therefore may not bind their targets as normal in a Y2H.
Researchers have depended on western blotting for decades to perform proteomic studies and validate many experimental results, including post-translational and CRISPR studies.
Western Blot has wide applications in biochemistry and other sciences because it can detect and characterize a wide variety of proteins. Western Blot is the process of separating proteins and identifying them in complex biological samples. The sensitivity of the process depends on the efficiency of protein transfer retention during processing and final detection. Western Blot works by using SDS-PAGE gel electrophoresis to separate proteins based on their size. Next, the protein is transferred to the membrane by an electric current. After the protein has been transferred, a primary antibody specific to the target protein is added. Next, the secondary antibody with the enzyme is applied, and finally, the substrate for the enzyme reaction bound to the secondary antibody is added for detection and visualization.
%20Analysis%20Service%2BKMD%20Bioscience3.jpg)
Fig 2: Flow chart of the immunoblotting experiment
Advantages of Western Blot:
(1) Simple equipment and inexpensive reagents allow Western Blot to be performed with only a small amount of reagents.
(2) Western Blot is widely used in many fields because it can detect not only proteins but also other properties of proteins.
(3) Sensitivity is the most significant advantage of Western Blot.
(4) Western Blot has higher specificity-the higher the specificity, the more independent the method is from the specificity of the antibody.
Limitations of Western Blot:
1) The tissue used in the experimental procedure must be homogenized.
2) The technology is an expensive process, the cost of antibodies and detection methods are expensive.
3) Small proteins may not be retained by the membrane during the experiment, while larger proteins are difficult to transfer to the membrane.
4) Some antibodies may have off-target effects by interacting with more than one protein in the sample.
3. Pull Down Assay Service
Pull-down assay is an in vitro method that is widely used for the confirmation or detection of physical interactions between two or more proteins. The principle of the pull-down assay is to utilize affinity ligands to capture interacting proteins. There are many types of tags for the bait of pull-down assay, such as GST, poly-His, Biotin, etc. The affinity ligands used to immobilize bait are glutathione, nickel, and chelate complexes, and streptavidin, respectively.
GST Pull-Down Assay Service:
The principle of pull-down assay is affinity purification, which is similar to immunoprecipitation, except that this method uses bait protein, instead of antibody to trap the target protein. In a pull-down assay, a bait protein is tagged and immobilized on beads with affinity ligand specific for the tag, thereby generating a "secondary affinity support" for purifying other proteins that interact with the bait protein. When incubated with a protein sample that contains putative "prey" proteins, such as a cell lysate, the bait can bind the prey, and thus trap the prey from the lysate.
%20Analysis%20Service%2BKMD%20Bioscience2.jpg)
Fig 3: GST pull-down experimental flow chart
Advantages of Pull-Down Assay:
(1) It has a wide range of applications, and label antibodies are often used to detect (such as GST).
(2) The operation is simple, and it is an effective method to directly detect the interaction between proteins.
Limitation of Pull-Down Assay:
1) The presence of a protein tag may influence the results of competition with the endogenous complex.
2) It doesn't reflect the interactions between proteins, because they don't necessarily meet spatially in the body, so it doesn't mean that they're bound physiologically.
4. Co-immunoprecipitation Service
Co-IP is a classic technology widely used for protein-protein interaction identification and validation. Based on the specific immunological interaction between the bait protein and its antibody, Co-IP has become an effective and reliable method for detecting the physiological interaction between proteins.
The principle of the Co-IP technique is as follows: many intracellular protein-protein interactions are retained when cells are lysed under non-denaturing conditions. The bait protein, for example, protein X, can be captured by its specific antibody stabilized to agarose beads. If there is another protein, the prey protein, for example, protein Y, binds to protein X in vivo, and the protein X - protein Y complex can then be precipitated together by the antibody. Subsequently, through the investigation of protein Y, we can confirm the protein X - protein Y interaction, or discover new interactors of protein X.
Advantages of Co-immunoprecipitation:
(1) Both the bait and prey proteins are in their native conformation in the Co-IP assay.
(2) The interaction between the bait and prey proteins happens in vivo with little to no external influence.
Limitation of Co-immunoprecipitation:
1) Low affinity or transient interaction between proteins may not be detected.
2) The result of Co-IP could not determine whether the interaction is direct or indirect since the possibility of involvement of additional proteins could not be ruled out.
How to Order?
If you have any questions regarding our services or products, please feel free to contact us by E-mail: [email protected] or Tel: +86-400-621-6806