Service Line:+86-022-82164980
Address:FL-4, Building A5, International Enterprise Community, Tianjin, China
Email:[email protected]
Inflammatory markers include heparin-binding protein (HBP), interleukin 6 (IL-6), serum amyloid A (SAA), serum C-reactive protein (CRP), and serum procalcitonin (PCT). Heparin-binding protein (HBP) is secreted by mature neutrophils and is a granule protein. Most of the HBP is found in azurophilic granules and a small amount is found in secretory vesicles. Compared with other biomarkers, HBP has the advantages of high sensitivity, specificity and detection at the early stage of infection.The relative molecular weight of mature HBP is known to be 24,000, and its structure contains 222 amino acids.HPB is a member of the serine protease family, has no proteolytic activity, and has a high affinity for the lipopolysaccharide Lipid A. It is a bactericidal and chemotactic protein. It is a multifunctional single chain glycoprotein with bactericidal and chemotactic properties.
KMD Bioscience successfully develops HBP antigen antibody diagnostic materials by utilizing its own experience and knowledge of structure-function of antibodies and proteins, combined with its mature bioinformatics prediction capability. Currently, KMD Bioscience's antibody platform includes four major platforms: monoclonal antibody production platform, polyclonal antibody production platform, phage antibody library technology platform, and antibody sequencing and application platform. All antibodies provided by KMD Bioscience are rigorously tested to ensure their purity and sensitivity for use in a variety of diagnostic platforms, such as LFIA, ELISA, CLIA, POCT, and so on.
The inventory of reagents associated with Heparin Binding Protein (HBP) that KMD Bioscience can offer:
|
CAT# |
Product Name |
Species |
Host |
Application |
Size |
Inquiry |
|
PA223 |
Human |
Mouse |
LFIA (Lateral-Flow Immunochromatographic Assay), CLIA (Chemiluminescence Immunoassay), ELISA |
1mg |
Inquiry |
|
|
PA224 |
Human |
Mouse |
LFIA (Lateral-Flow Immunochromatographic Assay), CLIA (Chemiluminescence Immunoassay), ELISA |
1mg |
Inquiry |
|
|
SMAG3259 |
Human |
Human WBC |
LFIA (Lateral-Flow Immunochromatographic Assay), CLIA (Chemiluminescence Immunoassay), ELISA |
1mg |
Inquiry |
HBP Structural Features:
Heparin-binding protein (HBP) is a member of the trypsin-like serine protease family, but HBP has no protease activity because the histidine residue at position 41 and the serine residue at position 175 have been replaced by serine and glycine residues, respectively. Nevertheless, HBP possesses a β-barrel structure that is characteristic of the catalytic center of the trypsin-like serine protease family. It consists of six antiparallel β-strands, so the ability of HBP to bind trypsin inhibitors (BPTIs) has not been lost.
The ability of HBP to bind endotoxin exists.Amino acid residues 20-44 in the HBP protein sequence form a v-type structure, and within this v-type region, the relatively exposed region of residues 25 phenylalanine, 26 cystine, 42 cystine, and 43 phenylalanine located at the apex of the v-type form a non-hydrophilic pocket structure, thus HBP has an affinity for the endotoxin lipid A component. affinity for the endotoxin lipid A component. This gives it the ability to bind endotoxin.
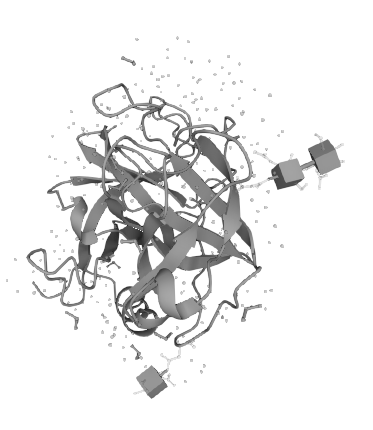
Figure 1 Schematic diagram of the molecular structure of HBP
Biological functions of HBP
Heparin binding protein (HBP) is secreted by polymorphonuclear leukocytes. It has excellent bactericidal properties, chemotactic properties and ability to regulate inflammation. Acts as an important granule protein. A large number of positively charged amino acid residues are present on the structure of HBP, while the EC surface is encapsulated by polysaccharides (with a large number of negative charges), so the polysaccharide encapsulation becomes a binding site for HBP binding on the EC surface. The combination of the two stimulates the Rho pathway of the small G protein family in the organism. This causes an increase in EC permeability and the release of large amounts of HBP, which causes the chemotaxis and activation of monocytes/macrophages, enhances their phagocytosis, and releases highly toxic oxygen radicals, which have a bactericidal effect. At this time, cytokines such as tumor necrosis factor-α (TNF-α) and ƴ-interferon (IFN-ƴ) were released in an increased manner, aggravating the inflammatory response. Increased expression levels of membrane proteins CD64 and CD32 increase the binding sites of IgG, producing an immune effect.
KMD Bioscience independently develops and produces heparin-binding protein products, by immunizing mice with HBP antigen to obtain mouse anti-human HBP monoclonal antibody (detection) and (capture), with high specificity and small batch-to-batch difference, which supports researchers to carry out the activity research of HBP protein targets and protein interaction research. And it can be applied to lateral flow immunochromatographic assay (LFIA), chemiluminescence immunoassay (CLIA) and enzyme-linked immunosorbent assay (ELISA). KMD Bioscience offers a diverse range of protein products, including recombinant proteins, viral proteins and bacterial proteins, etc. All the protein products provided have undergone rigorous QC validation and are characterized by low endotoxin content and high purity. With an experienced R&D team and mature quality management system, the platform can provide customized services according to customers' R&D needs, from gene sequence design, protein expression host selection, to monoclonal antibody production in every R&D process, to meet the needs of diagnostic raw material development, scientific research and other different applications.