Service Line:+86-022-82164980
Address:FL-4, Building A5, International Enterprise Community, Tianjin, China
Email:[email protected]
KMD Bioscience has been committed to protein interaction research for 10 years. Protein interactions can reveal the interaction patterns between molecules, effectively reveal the transduction process of signaling pathways in vivo, and are of great significance to the regulation of cells and their signals. Our research experts have accumulated many years of experience in protein interaction research and testing, and with mature technology, we can quickly complete the experimental content, saving valuable time and money for our customers. KMD Bioscience has rich experience in co-immunoprecipitation assay. Recombinant proteins, with GST, Myc-tag, Flag-tag, HA-tag and other tags can be efficiently expressed in both prokaryotic and eukaryotic expression systems. KMD Bioscience has sophisticated co-immunoprecipitation testing equipment and mature co-immunoprecipitation assay technology that are from Kyoto University, Japan. The co-immunoprecipitation assay and Pull Down technologies of KMD Bioscience come from Kyoto University's IPS laboratory in Japan, which ensures the accuracy of the experiments. KMD Bioscience, which insists on providing customers with high quality services, is able to provide one-stop technical services from recombinant protein preparation to pull down protein interactions detection to meet the needs of different customers.
Co-immunoprecipitation (Co-IP) assay technique is a classical method used to study protein-peptide and protein-protein interactions based on specific immunoreactivity between antibodies and antigens, and it is an effective method for determining the physiological interactions between proteins and peptides, and proteins and proteins in intact cells. The technique utilizes specific binding between antibodies and antigens, which specifically recognizes and binds to antigens in the sample, and then agarose cross-linked protein A/G specifically binds to the antibody, resulting in precipitation of the antigen-antibody complex. After elution and separation by gel electrophoresis, the amount of specific antigen in the sample is analyzed quantitatively or qualitatively by Western Blot. Immunoprecipitation verifies the natural binding of the two proteins in the cell, which is consistent with the actual situation in the body and provides high confidence in the results.
CoImmunoprecipitation assay experiments have many critical steps, in order to achieve the ultimate goal of the co-immunoprecipitation experiment, the immunoprecipitation system not only need high quality antibodies, also needs to be strict control indicators. KMD Bioscience can provide customers with high quality immunoprecipitation technology services. Researchers, in KMD Bioscience, strictly control the quality of each key step in the process to ensure that customers can get the desired results.
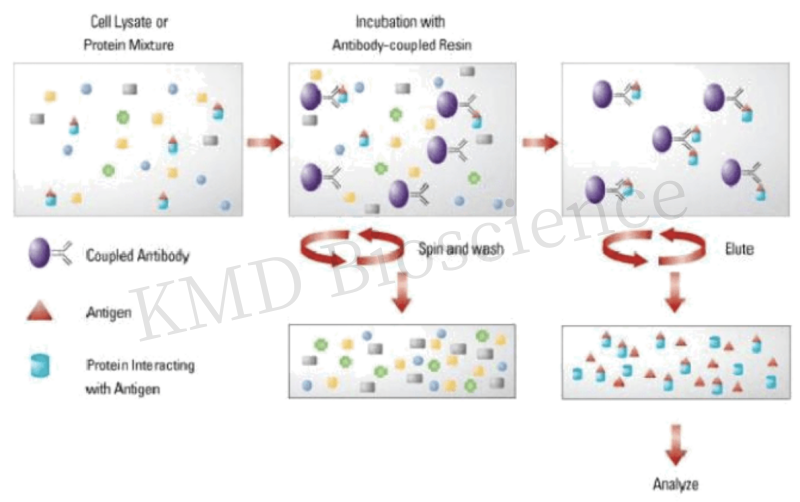
Figure 1 Principle of immunoprecipitation technique
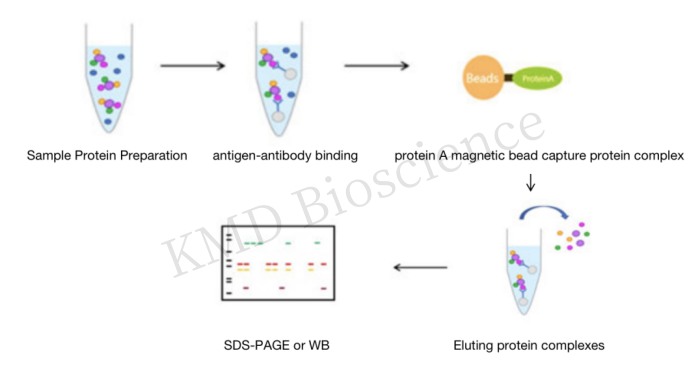
|
Co-immunoprecipitation Experiment |
|
|
Experimental principle |
*The experimental principle of the technique is that when cells are lysed under nondenaturing conditions, many of the protein-protein interactions present in intact cells are preserved. When protein A is immunoprecipitated with an antibody to protein A precured on agarose beads, protein B, which binds to protein A in vivo, is also precipitated; protein B is then detected by Western blot or LC-MS/MS, which demonstrates the interactions. |
|
Applications |
*Commonly used to verify the interaction of two known proteins *Search for the presence of unknown proteins interacting with known proteins in the cell |
|
Advantages |
*Interacting proteins are post-translationally modified and in their natural state *Protein interactions occur in their natural state, avoiding human influence *Interacting protein complexes can be isolated in their natural state |
F1: The destination strip is not detected or the signal is weak
A: 1. Low or no expression of the target protein.-------->Solution: The protein expression can be verified before the experiment, or the sample size can be increased; The protein lysate added to IP was increased and pre-treated.
2. The recognition epitopes of target proteins are blocked by interacting proteins.-------->Solution: Select antibodies with high affinity.
3. The target protein is not eluted.-------->Solution: Select the right strength and pH eluent,
4. the target protein is lost in the washing process.-------->Solution: Add appropriate detergent and NaCl.
5. Degradation of target protein. -------->Solution: Add protease inhibitors when handling samples, and all operations are carried out on ice to avoid repeated freezing and thawing of protein samples.
6. Selection of antibodies. -------->Solution: Replace the antibody or change the amount of antibody used to eliminate the problem of antibody failure.
F2: The background signal is too high
A2: 1. Degradation of target protein. -------->Solution: Add protease inhibitors when handling samples, and all operations are carried out on ice to avoid repeated freezing and thawing of protein samples.
2. The sample contains protein complexes.-------->Solution: Short ultrasonic sample, centrifuge and take supernatant for follow-up experiment.
3. Washing is not thorough. -------->Solution: Increase detergent and NaCl in the washing solution.
4. Non-specific proteins bind to magnetic beads.-------->Solution: Pre-treat with 1-5% BSA before use.
5, the antibody concentration is too high.-------->Solution: Select the appropriate antibody and adjust it to the appropriate concentration.
6. Poor specificity of antibody.-------->Solution: Replace the antibodies.
F3: False positives
A3: The phenomenon of non-specific binding (between magnetic beads, antibodies, and proteins) appears during the experiment. -------->Solution: Set up multiple control groups to exclude non-specific interference during verification.
F4: CO-IP verified that the two proteins interact, while other experiments verified the opposite results
A4: CO-IP is one technical means of verifying protein interaction, and the corresponding results weren’t verified by other technical means, which may be caused by the presence of intermediate proteins involved in the interaction of CO-IP.
F5: CO-IP verified that the two proteins don’t interact with each other, while other experiments verified the opposite results
A5: Co-IP assays could not detect weakly interacting or low expression protein.
F6: The Input stripe is clear, while the Co-IP stripe is weak
A6: If the interaction between two proteins is weak, or others are competing with the target protein.-------->Solution: The decoy protein can be re-tested as a target protein to increase the chance of interaction between the two proteins.
F7: There are no stripes for Input, but stripes for IP
A7: The protein may be degraded or the content is low.-------->Solution: A protease inhibitor is added to the sample while it is being processed, all on ice to prevent protein degradation and in addition, suggested that the loading amount of the input group be increased to ensure the accuracy and reliability of experimental results.
F8: Neither Input nor IP has stripes
A8: Protein degradation or low content.-------->Solution: Add protease inhibitors when handling samples, and increase the sample quantity.
If you have any questions regarding our services or products, please feel free to contact us by E-mail: [email protected] or Tel: +86-400-621-6806