2025-04-10 Hits(79)
Protein Purification
Overview of Protein Purification
Protein purification is an important step in biochemistry and molecular biology, which involves the process of separating a specific protein from a complex mixture, a complex process that may require multiple repetitions of different purification steps to achieve the desired high purity. After each step, purity and activity tests are needed to ensure the quality of the protein. The purpose of protein purification is to obtain high purity proteins for subsequent structural and functional studies or for the production of various biotechnology products.
The following are the general steps and methods of protein purification:
1. Sample preparation:
①Cell fragmentation: protein is released through the fragmentation of cells, including ultrasonic fragmentation, high pressure homogenization, freeze-thaw, etc.
②Removal of insoluble matter: Centrifugation to remove cell debris and unbroken cells.
2. Initial separation:
①salting out: by changing the salt concentration to precipitate the protein.
② isoelectric focusing: The use of proteins at their isoelectric point at the lowest solubility of the characteristics of separation.
3. Fine purification:
①Gel filtration chromatography (molecular sieve) : separation according to molecular size.
②Ion exchange chromatography: separation according to the charge properties of proteins.
③Affinity chromatography: The use of specific binding of proteins to specific ligands for separation, such as the use of antibodies, metal ion chelation, sugars, etc.
4. Purity detection:
①SDS-PAGE: The purity of protein was analyzed by polyacrylamide gel electrophoresis.
②HPLC: High performance liquid chromatography can further confirm the purity of the protein.
5. Protein activity detection:
Through specific enzymatic reaction or biological activity detection to determine the function of the protein.
Classification and Purification Strategy of Protein Purification
(1) Purification of recombinant protein
Recombinant proteins are proteins synthesized through genetic engineering techniques in a heterogenous expression system, usually a gene from another organism. Recombinant proteins can be mass-produced in E. coli, yeast, or mammalian cells. Recombinant proteins are usually expressed at high levels, sometimes forming inclusion bodies or aggregates. Impurities may include host cell proteins, nucleic acids, lipids, endotoxins, etc. Recombinant protein purification is generally easier because affinity labels and highly specific purification methods can be utilized [1].
Recombinant proteins are usually expressed by fusion affinity labels (such as His labels, GST labels, Myc labels, etc.), which allow for rapid purification using affinity chromatography. If the recombinant protein forms an inclusion body, it needs to be dissolved with a denaturant (e.g., urea, guanidine hydrochloride) and denaturated by gradually reducing the denaturant concentration. Since proteases may be present in host cells, protease inhibitors need to be added to prevent protein degradation.
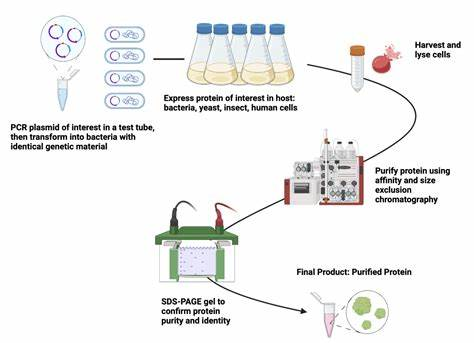
Figure 1:Schematic diagram of recombinant protein purification[2]
(2) Natural protein purification
Natural proteins are proteins produced in nature through the normal biosynthesis process of organisms. Natural proteins have very different properties, are directly extracted from animal or plant tissues, body fluids, or microorganisms, are usually expressed at low levels, and may have many different homologous proteins. Each protein is unique and comes with a rich background of impurities that may include other naturally occurring proteins, polysaccharides, lipids, pigments, etc. Because natural proteins do not contain any affinity labels, simple one-step purification is difficult to meet the needs, and natural proteins may have similar physical and chemical properties, making separation more complicated. Therefore, the natural protein is purified by ion exchange chromatography and molecular sieve chromatography, and the difference of various proteins (size, shape, charge, hydrophobicity, solubility and biological activity, etc.) is used to purify the target protein from the mixture, and the target product with high purity and biological activity is obtained.
Natural protein purification typically uses specific buffers and conditions to extract the target protein while preserving other proteins. Since there is no affinity label, purification may need to be achieved through a combination of multiple chromatography techniques such as ion exchange, gel filtration, affinity chromatography, etc. According to the physical and chemical properties of the target protein, different ion exchange chromatography is preferentially performed, different buffer systems are explored, and molecular sieve with appropriate pore size is selected for separation and purification. Small molecular proteins need to be purified by macroporous resin or reverse resin, further purified by HPLC, and finally confirmed by mass spectrometry identification, activity identification and other schemes. The target protein with high purity and biological activity was obtained. Natural protein purification also requires the addition of protease inhibitors to prevent protein degradation.
The Key Factors Affecting Protein Purification
When performing protein purification, the following factors need to be considered:
(1) Protein stability: Avoid conditions that will destroy protein stability.
(2) Contamination in the purification process: ensure that all reagents and equipment used are of high purity to avoid contamination.
(3) Repeatability of the experiment: ensure that the experimental steps can be repeated and the results are reliable.
(4) protease inhibitors need to be added to prevent protein degradation.
(5) Cell fragmentation, centrifugation, dialysis and other steps need to be gentle to avoid protein degradation or inactivation.
Verification of Protein Purification
(1) SDS-PAGE (Polyacrylamide gel electrophoresis) : The molecular weight of the protein can be observed and the purity can be evaluated by SDS-PAGE. The purified protein usually appears as a single band.
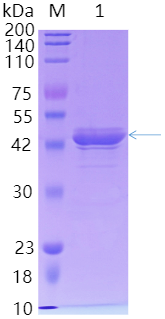
Figure 2:SDS-PAGE result of His label protein purification product
(2) Western blotting: Specific antibodies are used to detect the target protein, which can confirm the protein and assess its purity.
(3) Mass Spectrometry (MS) : mass spectrometry can accurately determine the molecular weight of the protein, and verify the identity of the protein through peptide mass fingerprint or amino acid sequence analysis.
(4) Ultraviolet spectroscopic analysis: The concentration and purity of the protein can be estimated by measuring the absorbance of the protein (usually at 280 nm).
Application of Protein Purification
Protein purification technology has a wide range of applications in many fields, with the deepening of scientific research and technological progress, purified proteins play an important role in more new fields and new applications.
(1) Basic research: protein purification technology can be used to study the structure and function of proteins, to understand their biological roles and regulatory mechanisms by purifying proteins, and to study the interactions and networks between proteins.
(2) Drug development: Protein purification can be used for the discovery and validation of drug targets, and the production of therapeutic proteins, such as insulin, growth factors, antibodies, etc.
(3) Diagnostic reagents: purified proteins are used in the preparation of diagnostic kits, such as ELISA, Western blot, etc., for the detection of disease markers and disease diagnosis.
(4) Biotechnology: In protein engineering, purified proteins are used to construct and screen mutant libraries, and protein purification can also be used to optimize protein expression systems.
References
[1] Park J W , Lanier T C .Scanning Calorimetric Behavior of Tilapia Myosin and Actin due to Processing of Muscle and Protein Purification[J].Journal of Food Science, 2010, 54(1):49-51.DOI:10.1111/j.1365-2621.1989.tb08564.x.
[2] Kapoor M .How to purify proteins[J]. 2006.
[3] Judge R A , Johns M R , White E T .Protein purification by bulk crystallization: The recovery of ovalbumin[J].Biotechnology & Bioengineering, 2010, 48(4):316-323.DOI:10.1002/bit.260480404.