2024-02-18 Hits(239)
Single B Cell Screening technology
Monoclonal antibodies play a key role in the fight against the novel coronavirus and have been shown to be effective in reducing the progression of mild to severe symptoms in patients. However, future progress in this field depends on the timing and technology of antibody development. A recent article published in the journal Nature-Scientific Report demonstrates the use of single B cell clonal screening for rapid and reliable identification of high-affinity, potent neutralizing antibodies to the novel coronavirus (SARS-CoV-2) and explains how to enhance neutralizing activity.
After vaccination, different types of antibodies are generally produced, taking neutralizing antibodies as an example: neutralizing antibodies are antibodies produced by the organism stimulated by the outermost envelope or capsid antigen of the virus that can bind to the virus and make it lose its infectiousness. In the current research on COVID-19 vaccine, neutralizing antibodies belong to the focus of research, such as the receptor-binding domain (RBD) for S1 protein and the N-terminal domain (NTD) for S1 protein.
The neutralizing antibody studied in this study belongs to the former. For the S1 protein receptor binding domain (RBD), it can bind to the RBD region of SARS-CoV-2 and block the binding of this region to the ACE2 receptor, so as to prevent the virus from invading cells. This type of antibody is the most produced by the human body and the most important type of antibody that has a good effect on blocking the virus.
Antibodies can be found faster through single B cell screening. The antibody discovery process can be shortened to a few weeks through single B cell sorting. This pioneering approach involves isolating live cells from immunized animals or rehabilitated patients, classifying antigen-specific cell subpopulations, and recovering paired VH/VL antibody genes by RT-PCR and PCR; The encoded antibodies can be expressed and characterized to identify good performing clones, as shown in Figure 1.
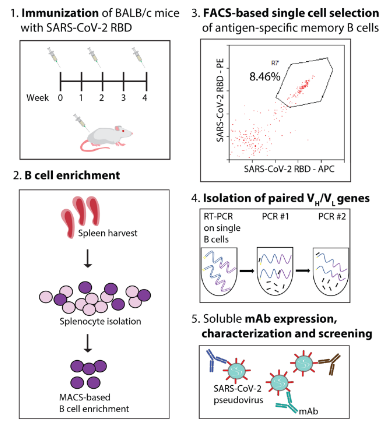
Figure 1: A review of methods for isolating and identifying effective SARS-CoV-2 neutralizing antibodies from single B cells
The advantage of this method is that it uses the native antibody sequence with high somatic mutation and preserves the native VH/VL pairing. In addition, the discovery process is high-throughput, allowing further evaluation of the highly immune antibody library.
To evaluate the effectiveness of single B cell screening in isolating neutralizing antibodies against SARS-CoV-2 virus, we prepared a recombinant form of SARS-CoV-2 receptor-binding domain (RBD) in HEK293T cells and purified its protein.
After immunizing BALB/c mice, anti RBD IgG1+B cells were isolated from spleen material by single cell sorting, and the VH /VL gene was treated. The resulting VH/VL gene pairs were cloned into mammalian expression plasmids (including the constant region of human IgG1 and the kappa light chain region for chimeric antibody expression) and transfected into HEK 293-6E cells instantaneously. Antibody identification includes the evaluation of Protein A magnetic beads' purification, antigen-binding ability, neutralizing activity and other properties.
After the recombinant antibody is produced, its affinity must be analyzed for downstream applications. Antibody affinity detection methods include biofilm interferometry (BLI), solid phase radioimmunoassay (SP-RIA), equilibrium dialysis, binding antigen precipitation, radioimmunoassay (RIA), ELISA, and surface plasmon resonance (SPR).
RBD regions of SARS-CoV or SARS-CoV-2 with His labels were incubated separately with Dynabeads magnetic beads to detect the specificity of neutralizing antibodies. The two magnetic beads were incubated separately with different concentrations of antibodies, and the unbound magnetic beads were eluted. It was then incubated with fluorescent secondary antibody (goat-derived, anti-human IgG, Fc fragment specific affinity purified secondary antibody, Jackson ImmunoResearch) and analyzed by flow cytometry.
B-cell screening has been shown to rapidly identify high-affinity, potent SARS-CoV-2 neutralizing antibodies. In addition, its use in combination with antibody modification is expected to be an effective strategy to prevent COVID-19.
KMD Bioscience has rich experience and strict quality control system in single B cell monoclonal antibody production services, and can provide customers with personalized customized services to meet the actual needs of customers. Join KMD Bioscience on the forefront of biotechnological innovation. Explore the unparalleled possibilities that our service bring to your research, and let’s together pave the way for a future of groundbreaking discoveries in immunology and beyond.