2024-11-19 Hits(551)
In the biomedical field, yeast surface display technology has become an important tool for the discovery of novel antibodies and biomolecules. KMD Bioscience's yeast display library screening service utilizes yeast cell surface display of target molecules to rapidly identify candidate molecules with high affinity and specificity through high-throughput screening. Our services cover a wide range of aspects such as antibody yeast display and nanobody screening, providing one-stop solutions for our customers.
KMD Bioscience's yeast surface display library screening service integrates advanced genetic engineering, protein engineering and high-throughput screening technologies, realizing a revolutionary leap in antibody screening. Our service is based on the construction of high-quality yeast display libraries, combined with highly efficient screening strategies and sophisticated analytical tools, to ensure that antibody candidates with specific binding activity and high affinity are accurately screened from a large number of antibody fragments. This process not only significantly improves screening efficiency and accuracy, but also effectively reduces R&D costs and time costs.
KMD Bioscience's yeast surface display library screening service is based on well-designed yeast display vectors and optimized screening process. We clone the antibody gene sequences provided by our customers into yeast display vectors and introduce them into yeast cells through efficient transformation techniques to construct libraries containing rich antibody diversity. Subsequently, using advanced flow cytometry or magnetic bead screening technology, the antibodies in the library are screened on a large scale and with high precision to identify antibody candidates that meet specific requirements, which can be used in new drug development, disease diagnosis, vaccine design and other avenues.
Yeast Display Library Screening Service
With the deepening of life science research, antibody drugs have become increasingly prominent in the treatment of diseases. However, traditional antibody screening methods are limited by factors such as screening throughput, cost and cycle time, which are difficult to meet the rapid needs of modern drug discovery and development. Yeast surface display library screening technology has emerged to provide strong technical support for the rapid discovery and validation of antibody drugs.
Yeast surface display technology has become a powerful tool for protein engineering, especially antibody engineering. Using yeast surface display technique to screen single chain antibody fragments from scFv libraries is a successful alternative to classical hybridoma technique. Yeast display technology can be combined with flow cytometry to directly analyze the expression, stability and affinity of display proteins, thus becoming an efficient library screening system. Yeast surface display technology has been widely used for screening and optimization of single chain antibodies (scFv) against various antigens. Through library screening with these techniques, antibody fragments with similar affinity can be generated without immunizing animals, and the resulting antibody fragments can also interact with autoantigens or toxic molecules[1]. Compared to phages, yeast is a powerful genetic engineering tool that has all the beneficial advantages of eukaryotic systems, such as post-translational modification and protein quality control.
The Principle of Yeast Display Library Screening
There are two kinds of yeast surface display system commonly used: lectin display and floc display. The mating protein A-agglutinin system of Saccharomyces cerevisiae (composed of two subunits of AgA1-AGA2 linked by disulfide bonds) fused the target protein to Aga2 by cloning the target protein on AGA2-containing vector, and introduced the vector containing Aga1 (covalentially linked to the yeast cell wall β-glucan). Aga2 and Aga1 recognize and bind via disulfide bonds, thus displaying the target protein on the cell surface.
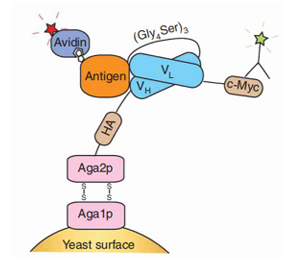
Figure 1: Schematic representation of yeast surface display [2]
In yeast surface display, the target protein is expressed on the cell surface by fusion with the yeast cell wall protein Aga2, and the fusion protein is easily recognized by large molecular proteins such as antigens. It also has the unique advantages of eukaryotic post-translational modifications, which can fold and secrete large and complex glycosylated target proteins. In the yeast display library, each yeast cell can express about 50,000 purpose-specific proteins. The high throughput of the surface display fusion protein and the size of the yeast cell allow the yeast surface display platform to be well integrated with flow cytometry, so that the combination library can be quantitatively screened and the binding relationship between different target proteins (binders) and the target can be finely distinguished (i.e. fine affinity recognition). In addition, flow cytometry can easily detect biophysical characteristics such as the stability of the yeast display protein-target interaction without protein degradation and protein secretion and expression purification. Therefore, yeast surface display is a good display platform for eukaryotic proteins or polysulfide proteins.
Yeast Display Library Screening Service Process
1. Library construction stage: Using genetic engineering technology, the antibody gene fragments are cloned into the yeast display vector and introduced into the yeast cells through electroporation and other efficient transformation techniques to construct a high-quality yeast display library.
2. library amplification stage: the yeast library is amplified and cultivated under appropriate culture conditions to ensure the stable maintenance of library capacity and diversity.
3. High-throughput screening stage: The antibodies in the yeast libraries are screened with large-scale and high-precision using advanced flow cytometry or magnetic bead screening technology to identify antibody candidates with specific binding activity.
4. Candidate validation stage: Using biochemistry and molecular biology, the screened antibody candidates are rigorously validated for specificity, affinity and other properties.
5. Data analysis and report preparation: Integrate the screening and validation data, use bioinformatics tools for in-depth analysis, and prepare detailed screening reports and data analysis reports.
In general, magnetic screening and FACS screening are used to screen scFv and identify scFv affinity in yeast surface display technology. First, in magnetic screening, yeast cells expressing scFv libraries are incubated with micron magnetic beads coated with the target (antigen), and then the yeasts and magnetic beads mixture are separated by magnets. Due to the strong interaction between yeast and magnetic beads, scFv fragments with low affinity to the target can also be separated. Then, the yeast cells obtained after magnetic screening were further screened by flow cytometry to isolate and collect scFv library cells with higher affinity to the target (antigen).
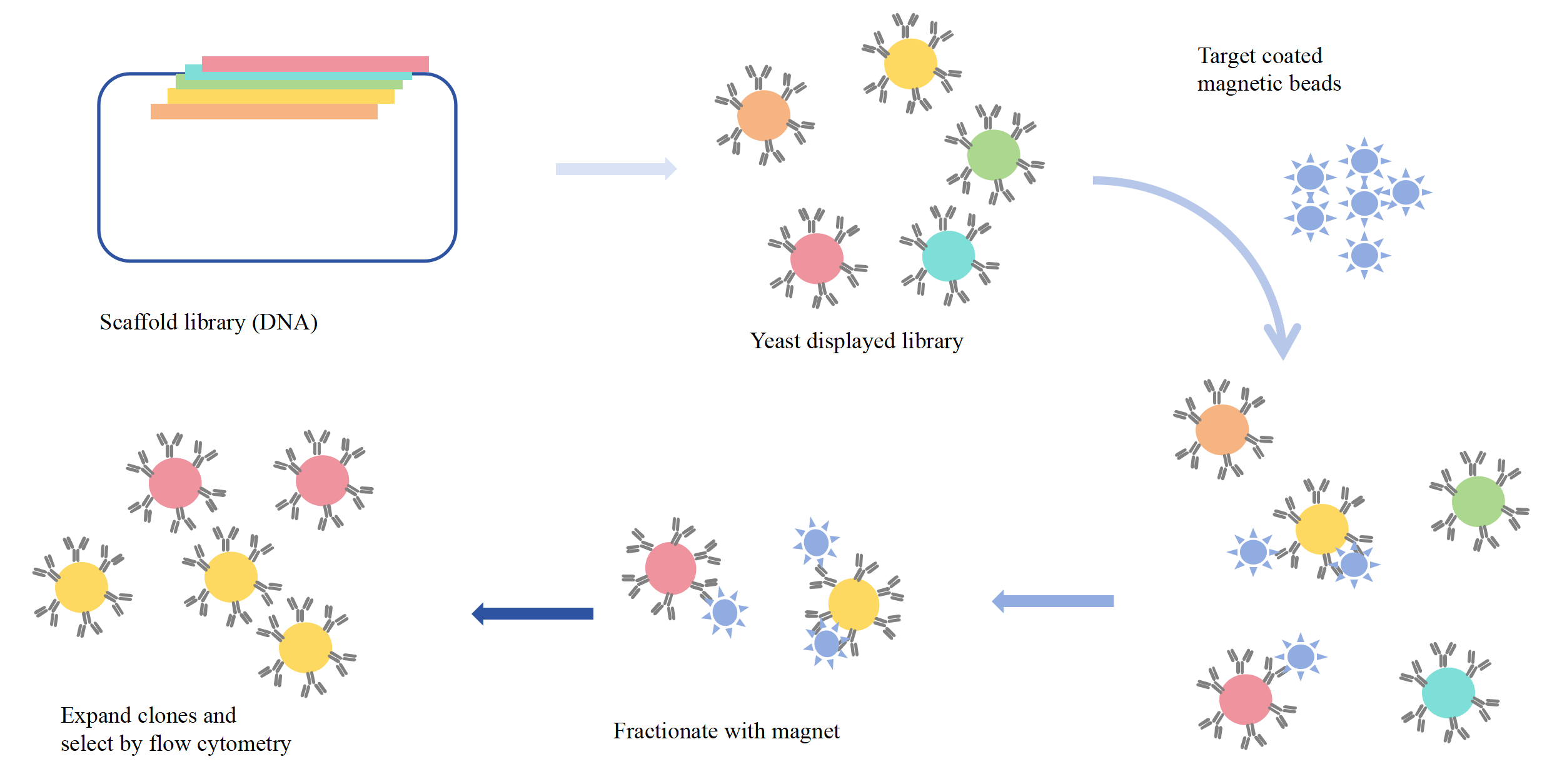
Figure 2: Yeast surface display process
Yeast Display Library Screening Service Workflow
| Service Steps |
Service Content |
QC Standards |
Timeline |
Delivery Standards |
|
Requirements analysis |
Define customer requirements and project objectives |
Clarify customer requirements |
1-2 working days |
Requirements analysis report |
|
Project design |
Customized experimental plan |
Reasonable and feasible plan |
3-5 working days |
Documentation of experimental plan |
|
Vector construction |
Construct yeast display vector |
Vector correctness verification |
1-2 weeks |
Constructed vector |
|
Yeast transformation and screening |
Transform yeast cells and screen |
Transformation efficiency and screening accuracy |
2-3 weeks |
Screening report |
Advantages of Yeast Display Library Screening Service
.png)
Reference
[1] Klimka A, Matthey B, Roovers R C, et al. Human anti-CD30 recombinant antibodies by guided phage antibody selection using cell panning[J]. Br J Cancer. 2000, 83(2): 252-260.
[2] Chao G, Lau W L, Hackel B J, et al. Isolating and engineering human antibodies using yeast surface display[J]. Nat Protoc. 2006, 1(2): 755-768.
[3] Gera N, Hussain M, Rao B M. Protein selection using yeast surface display[J]. Methods. 2013, 60(1): 15-26.
[4] WELLNER A, MCMAHON C, GILMAN MSA, CLEMENTS JR, CLARK S, NGUYEN KM, HO MH, HU VJ, SHIN JE, FELDMAN J, HAUSER BM, CARADONNA TM, WINGLER LM, SCHMIDT AG, MARKS DS, ABRAHAM J, KRUSE AC, LIU CC. Rapid generation of potent antibodies by autonomous hypermutation in yeast[J]. Nature Chemical Biology, 2021, 17(10):1057-1064.
[5] GRAY A, BRADBURY ARM, KNAPPIK A, PLÜCKTHUN A, BORREBAECK CAK, DÜBEL S. Animal-free alternatives and the antibody iceberg[J]. Nature Biotechnology, 2020, 38(11):1234-1239.
[6] BOWLEY DR, LABRIJN AF, ZWICK MB, BURTONDR. Antigen selection from an HIV-1 immune antibody library displayed on yeast yields many novel antibodies compared to selection from the same library displayed on phage[J]. Protein Engineering, Design and Selection, 2007, 20(2):81-90.
Recommended Services
Antibody Humanization Service: Converting non-human antibodies into human antibodies to reduce immunogenicity.
Antibody Affinity Maturation Service: Improve the affinity of antibodies and enhance their biological functions.
CAR-T Cell Therapy Services: Develop CAR-T cell therapy strategies using screened antibodies.
Vaccine Development Support: Provide technical support and services for vaccine design and development.
How to Order?
If you have any questions regarding our services or products, please feel free to contact us by E-mail: [email protected] or Tel: +86-400-621-6806