Service Line:+86-022-82164980
Address:FL-4, Building A5, International Enterprise Community, Tianjin, China
Email:[email protected]
GST stands for Glutathione S-transferase. It is a 211 amino acid protein (26 kDa) whose DNA sequence is frequently integrated into expression vectors for the production of recombinant proteins. GST is typically fused to the N-terminus of a recombinant protein through recombinant DNA technology. GST fusion protein has been widely used in recombinant protein expression and production. The fusion of the GST protein allows simple and efficient affinity purification of the GST-tagged protein using Glutathione affinity agarose resin. The GST-tag also can be used for detection in western blot by using a GST-tag-specific antibody.
Polyhistidine tag, also known as His tag, is an amino acid motif in proteins, typically composed of at least six histidine (His) residues located at the N-terminus or C-terminus of the protein. When a protein with a His tag comes into contact with a carrier fixed with metal ions such as nickel, the histidine residue will chelate the metal ion and bind to the carrier. Due to other proteins not binding to the carrier or having weak binding strength, they can be removed by washing the carrier with an appropriate buffer solution.
Supported by various types of protein purification assay services, scientists in KMD Bioscience insist on providing high-quality His/GST tag purification services, including detailed information consultation, customizable experimental design, and efficient implementation.
GST Protein Purification Service Process
.png)
Fig1 GST tag protein purification process.
.png)
Fig2 GST tag protein purification detail protocol.
His Tag Purification Service
Many recombinant proteins are expressed as fusion proteins, meaning that they contain an affinity/epitope tag (e.g. His or GST). His tag purification (Fig 1) uses affinity chromatography to capture recombinant proteins with 4–10 histidine residues. His tag proteins can be purified from several expression systems under native or denaturing conditions. The main drawback is that the technique often requires optimization to minimize the nonspecific binding of host cell proteins. The protein binding capacity of resins is typically 10–40 mg/mL. The purity can be 95% or higher in KMD Bioscience's lab.
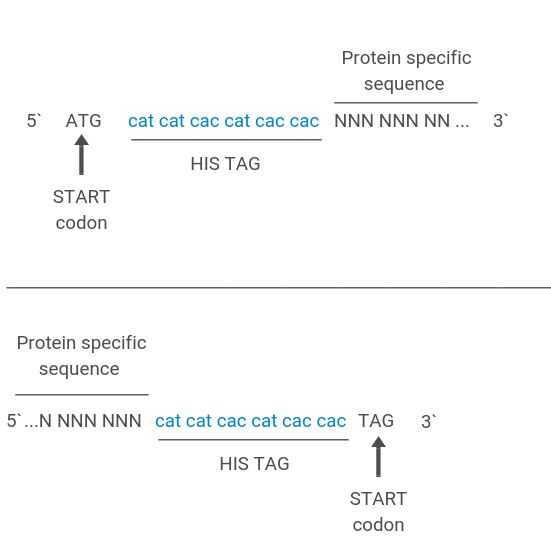
Fig3 His tag fused to the N or C terminus of recombinant proteins.
His tag purification can be used as the only purification step for applications that do not require proteins with extremely high purity. His tag purification can be used to purify inclusion bodies as well. His-tagged proteins can be purified by single-step affinity chromatography, namely immobilized metal ion affinity chromatography (IMAC), which is commercially available in different formats, with Ni-NTA matrices being widely used. IMAC purification can equally be performed in prepacked columns using FPLC or other liquid chromatography instrumentation or using magnetic bead-based methods (Block et al., 2009). The histidine tag is small, so it usually does not have to be removed.
Remove the tag if it interferes with the function of the target protein or if the target protein needs to be in a native state (e.g., for structural studies). Cleave the tag using a protease that recognizes the protease recognition sequence in the target protein. Some recombinant proteases are histidine-tagged and can be easily removed after a second pass over the resin used to purify the protein of interest.
All proteins purified by KMD Bioscience are analyzed by SDS-PAGE gel to maintain high purity and integrity. All corresponding data will be provided to the customer. For other available analyses, please contact us today if you are interested in His/GST tag purification service.
How to Order?
If you have any questions regarding our services or products, please feel free to contact us by E-mail: [email protected] or Tel: +86-400-621-6806