Service Line:+86-022-82164980
Address:FL-4, Building A5, International Enterprise Community, Tianjin, China
Email:[email protected]
Background
For the first time, Dr. H. zur Hausen of the University of Heidelberg in Germany, on Feb. 12, 1985, introduced human papillomavirus (HPV). HPV are small, nonenveloped and double-stranded DNA viruses that infect both mucosal and cutaneous epithelial cells. Its genome encodes six early regulatory proteins (E1, E2, E4, E5, E6, and E7) and two late structural proteins(L1 and L2). Genital HPV infection is the most common sexually transmitted infection worldwide, involving 75% to 80% of men and women of all ages. HPV has been recognized as the cause of around 5% of all cancers worldwide [1].
As of September 2023, 231 HPV types have been identified based on differences in the DNA sequence of the L1 capsid protein. The two HPV types, HPV16 and HPV18, have been identified as the most prevalent types associated with cervical cancer and are accountable for 70% of cervical cancers and precancerous cervical lesions. HPV lesions arise from the unchecked cell proliferation and mutations and lead to cancer ultimately. Due to the rapid immune clearance, most HPV infections do not cause symptoms and resolve spontaneously within 1 or 2 years; persistent high-risk HPV infection has been shown to be involved in the increased risk of high-grade cervical intraepithelial neoplasia and cancer. The HPV 9-valent recombinant vaccine can prevent 90% of cervical cancers and 90% of genital warts.
The prophylactic vaccines activate the humoral immunity and production of virus-neutralizing antibodies, inhibit viruses from entering into host cells, and induce effective protection against HPV infection. Three prophylactic licensed vaccines for the prevention of high-risk HPV infection are available in most countries: Gardasil, Cervarix, and Gardasil-9. These vaccines were produced by recombinant DNA technology using the HPV L1 capsid proteins, which self-assembled into the noninfectious form of virus-like particles (VLPs). The HPV 9-valent recombinant vaccine can prevent 90% of cervical cancers and 90% of genital warts.
.png)
Figure 1 The production mechanisms of prophylactic vaccines. [2]
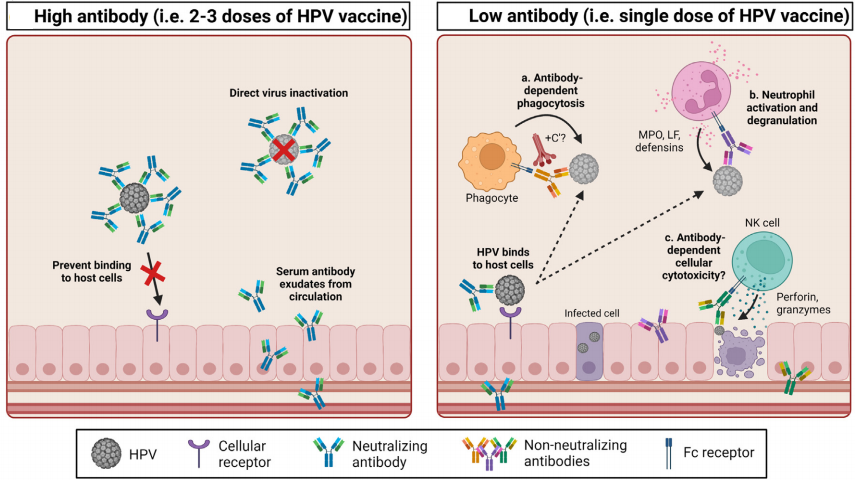
Figure 2 Potential mechanisms of antibody-mediated inhibition of HPV infection [3]
High concentrations of neutralizing antibody following two or three doses of HPV vaccine directly inactivate HPV and prevent virions from binding to keratinocytes (i.e., neutralization). Lower concentrations of neutralizing antibody following single-dose HPV vaccination might only partially prevent HPV from binding to host cells and require clearance by non-neutralizing antibody functions such as antibody-dependent phagocytosis (with or without complement, C’), neutrophil activation and degranulation of antimicrobial proteins. Antibodies induced by HPV vaccination may also mediate the clearance of infected epithelial cells by cellular cytotoxicity.
KMD Bioscience provides the following three high-quality humanized HPV neutralizing antibodies for our customers. The antibodies have been tested by ELISA and neutralization assay, and are suitable for other applications.
|
Product Cat. |
Product Name |
|
MA2624 |
|
|
MA2625 |
|
|
MA2626 |
Please feel free to contact us for more information!
Reference
[1] Wang R, Pan W, Jin L, et al. Human papillomavirus vaccine against cervical cancer: Opportunity and challenge[J]. Cancer letters, 2020, 471: 88-102.
[2] Yousefi Z, Aria H, Ghaedrahmati F, et al. An update on human papilloma virus vaccines: history, types, protection, and efficacy[J]. Frontiers in Immunology, 2022, 12: 805695.
[3] Quang C, Chung A W, Frazer I H, et al. Single-dose HPV vaccine immunity: is there a role for non-neutralizing antibodies?[J]. Trends in Immunology, 2022, 43(10): 815-825.