2024-02-19 Hits(291)

Introduction to VHH Nanobody Platform
|
KMD Bioscience has a mature recombinant antibody library construction system to provide customers with high quality VHH naïve and immune library construction services. We construct VHH libraries with good diversity and large library capacity of more than 1x 10^9 CFU/mL, which helps to facilitate our customers' research and program development. VHH, also known as nanobodies, have a structure containing only the heavy chain antibody variable region and are only 12-15 kDa in size, much smaller than conventional and other forms of recombinant antibodies. VHH has the advantages of good stability and high penetrability, which can penetrate the blood-brain barrier, and has a broad application prospect in the fields of medical diagnosis, antibody drug KMD Bioscience not only provides customers with high-quality VHH library construction services, but also has a complete antibody library screening system of immunity, and we are committed to producing specific and stable VHH antibodies for our customers based on advanced phage, yeast cells and other display technologies.
|
|
The Procedure of VHH Nanobody Library Construction Service
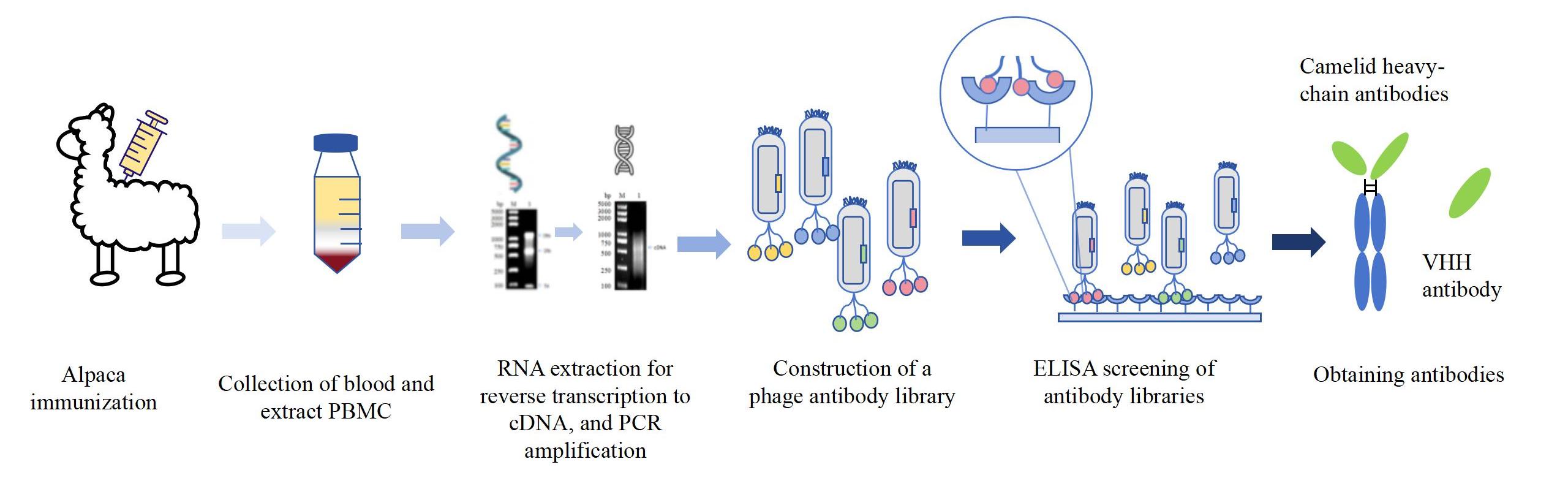
Related Services of VHH Nanobody Platform
KMD Bioscience not only provides customers with high-quality VHH library construction services, but also has a complete antibody library screening system. In addition, our laboratory platform is well-equipped and our researchers have rich experience in recombinant antibody expression, purification and sequencing, etc., which can provide customers with complete scientific research services and save a lot of experimental costs and time.
Recommended Special Services
KMD Bioscience can provide customers with antibody-related services, and its technology platform provides customers with higher quality services.
 |
 |
 |
|
|
|
|
VHH Nanobody Platform Related Technical Service Resources
We are willing to share with you the rich technology and resources used in VHH library related technical services to solve your doubts. If you need to view more, please refer to our related articles.
|
|
|
|
|
|
|
|
FAQ-VHH Nanobody Production
1. What is a VHH antibody?
A: VHH antibody is a natural missing light chain (VL) antibody found in the serum of camel animals. VHH antibody structure is simple and consists of only two heavy chains (VH). Nanobodies comprise heavy chain variable region (VHH) with a molecular weight of about 15 kDa. VHH antibody has the normal ability to recognize antigens and shows excellent affinity, excellent specificity, excellent stability, and excellent penetration. Antibodies to the structure have since been found in other animals, such as alpacas and sharks. Nanobodies, which contain only one heavy chain variable region and two conventional CH2 and CH3 regions, despite having no VL domain, are a highly stable single-domain antibody and the smallest known binding unit with antibody activity. KMD Bioscience can provide customers with including nano antibody preparation, nano antibody library construction and screening, VHH nano antibody construction, and a series of services, through the nano antibody synthesis and phage display development platform, it can efficiently build and screen including a variety of different types of phage antibody library, provide our customers with specific, high specificity of antibody solutions. We can also develop professional antibody humanization strategies for our clients, which can be humanized for Nanobodies, and our company is almost equivalent to human antibodies.
2. What are the main aspects of the VHH antibody discovery service?
A: First, the alpaca without any disease, allows the immunogens to stimulate its immune system to produce an immune response, and these animals can produce single-domain antibodies. Then, we isolated single B cells from alpaca PBMC, and RNA from B cells was extracted and transcribed into cDNA. Finally finally used as a template and screened our target VHH sequence by electrophoresis. Next, we performed a sequencing analysis of the screened VHH antibody sequence, which can improve the stability and affinity of the prepared VHH antibody. At the same time, we can also conduct site-directed mutagenesis and stability screening of VHH antibodies. We can obtain higher stability of VHH antibody mutants by introducing special methods such as certain specific mutations for screening. The engineer then inserted the resulting VHH sequence into the appropriate expression vector (such as plasmids, viruses, etc.) for VHH antibody expression. Finally, through phage display technology or yeast display technology, VHH antibodies are expressed on the host surface, and then Nanobodies that can specifically bind the target antigen are selected by using coated antigens and ELISA. KMD Bioscience also sequenced and verified the selected Nanobodies to ensure the correctness and functional activity of the nanobody sequences delivered to customers.
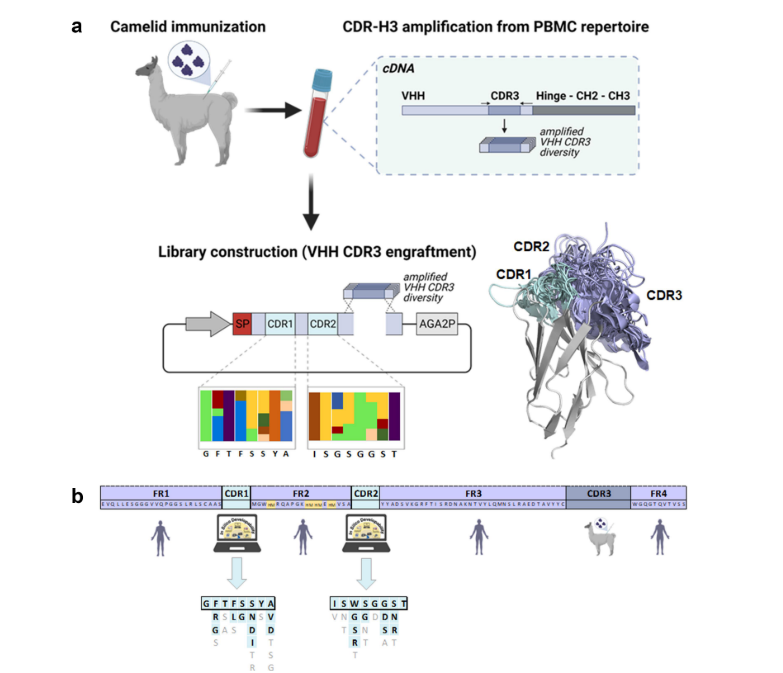
Fig: Flow chart of antibody to VHH discovery. (Reference source: Arras P, Yoo HB, Pekar L, Schröter C, Clarke T, Krah S, Klewinghaus D, Siegmund V, Evers A, Zielonka S. A library approach for the de novo high-throughput isolation of humanized VHH domains with favorable developability properties following camelid immunization. MAbs. 2023 Jan-Dec;15(1):2261149. )
3. Advantage of the VHH antibody discovery services?
A: VHH technology takes advantage of various advances in genetic engineering, so that the target gene is respliced and rearranged together under normal circumstances, which can provide a highly binding tool for other technologies, which can contain various tags, and the antibodies produced by VHH technology are convenient for purification. Using high-throughput screening techniques, KMD Bioscience can screen VHH antibodies with specific functions required by clients from a large number of antibodies in a short period. Meanwhile, VHH antibodies can be produced indefinitely and economically, when VHH antibodies are exposed to high temperatures and other solvents. VHH antibodies can be genetically manipulated to generate other uses, such as becoming scaffolds, labeling, and changing specific amino acids. VHH antibodies are suitable for any common platform using conventional antibodies, such as microtiter plates, electrochemical biosensors, and lateral flow devices. Due to the smaller size of VHH antibodies can have a higher density in the binding domain, such that VHH antibodies have an outstanding advantage in increased signal and therefore higher sensitivity. At the same time, VHH antibody has a very broad application in tumor diagnosis and treatment, inflammation diagnosis, the treatment of central nervous system diseases, and other fields. VHH antibodies are particularly useful for monitoring mycotoxins in food and feed because they are easily genetically engineered and have excellent stability.
4. How do we choose the immunogens?
A: In the process of immunization, we use a variety of immunogens. According to the properties of immunogens, we can be them into natural antigens, recombinant antigens, synthetic antigens, and small molecule antigens. Natural antigens include viral immunogens. The purification process of natural immunogens is complicated, which is also more difficult than other immunogens, and the purification cost is also higher. The viral immunogen is divided into whole virus inactivated vaccine, subunit vaccine, viral vector vaccine, and mRNA vaccine. The whole virus-inactivated vaccine can stimulate the body to produce an immune response, but the virus needs to be completely inactivated, and the preparation process is more complicated. For the subunit vaccine, we only used the viral surface protein as the antigen, so the safety of this immunogen is high, but the adjuvant needs to be added to enhance the immune effect. After genetic modification, adenovirus and lentivirus are used as vectors to induce the occurrence of immune response through viral replication and expression. The immune effect is efficient and lasting, but the preparation process is complex, so we need to strictly control biosafety. While mRNA can be directly imported into cells, so that cells can express antigen and stimulate the occurrence of immune response, this process is more difficult, which requires us to ensure the stability and delivery efficiency of mRNA. Although recombinant antigens differ in conformation from natural antigens, they can undergo extensive industrial production. Small molecule proteins or polypeptide antigens, can be prepared by in vitro synthesis methods. Their structure is controllable, but it can be complicated in design. Small molecule antigens are mostly small molecule compounds such as peptides and nucleotides, because they are not immunogenic by themselves, so they can only be used as immunogens after connection with macromolecular carriers.
5. Advantages of the VHH structure?
A: The molecular weight of Nanobodies is very small, usually around 12-15 kDa, only one-tenth of the traditional IgG antibody. The crystal structure is a rugby ball about 2.5nm in diameter and about 4.2nm long. The unique molecular structure allows for good tissue penetration, shorter half-life, and higher renal clearance, through the blood-brain barrier. The Nanobodies consist of complementary determining and backbone regions. Complcomplementary decision regions include CDR 1, CDR 2, and CDR 3. The length range of 3 to 28 amino acids in the CDR 3 region ensures the storage capacity of the nanobody library. Compared with the traditional antibody CDR 3 region of only 8 to 15 amino acids, some of the CDR 3 regions with longer CDR 3 helped us to identify hidden epitopes on the surface of the antigen. The backbone region of the Nanobodies includes FR 1, FR 2, FR 3, and FR 4, with four hydrophilic amino acid mutations on FR 2, and this mutation enhances the water solubility of the antibody. The special disulfide bond between CDR 1 and CDR 3 enhances the stability of antibodies under high pressure, high temperature, denaturant, and other conditions, which is conducive to the production and preservation of Nanobodies, and also creates the possibility of new administration methods.