Service Line:+86-022-82164980
Address:FL-4, Building A5, International Enterprise Community, Tianjin, China
Email:[email protected]
KMD Bioscience is experienced in developing custom anti-idiotypic antibodies (anti-idiotype antibody, or anti-id). The anti-idiotypic antibodies produced by KMD Bioscience have high specificity and affinity to support your research, like preclinical research, clinical trials, and biosimilar antibody development research. In drug development, it can be used for pharmacokinetic (PK) determination of antibody-drug levels in vivo, as well as for anti-drug antibody (ADA) detection to detect immune responses to therapeutic antibodies.
Pharmacodynamics (PK) refers to the trend of drug concentration in the blood over time, reflecting the degree of drug absorption and conversion. In pharmacokinetic studies, anti idiotypic antibodies can specifically recognize the unique location of biological drugs and are important detection reagents for PK research. The presence and level of anti-drug antibodies (ADA) are considered as a measure of the immunogenicity of biological therapeutic agents. ADA can affect the pharmacodynamics and pharmacokinetics of its target drug, thereby reducing its efficacy.
Anti-Idiotypic Antibody Production Service
An antibody possesses several complementarity-determining regions (CDRs). Anti-idiotypes are typically raised against the CDR regions (idiotype) of the biosimilar antibody. Therapeutic antibody biosimilars (ADC biosimilar) are usually coupled to drugs using CDR1 or CDR3 domains isolated from the variable regions of antibodies and used as a cancer immunotherapy tool.
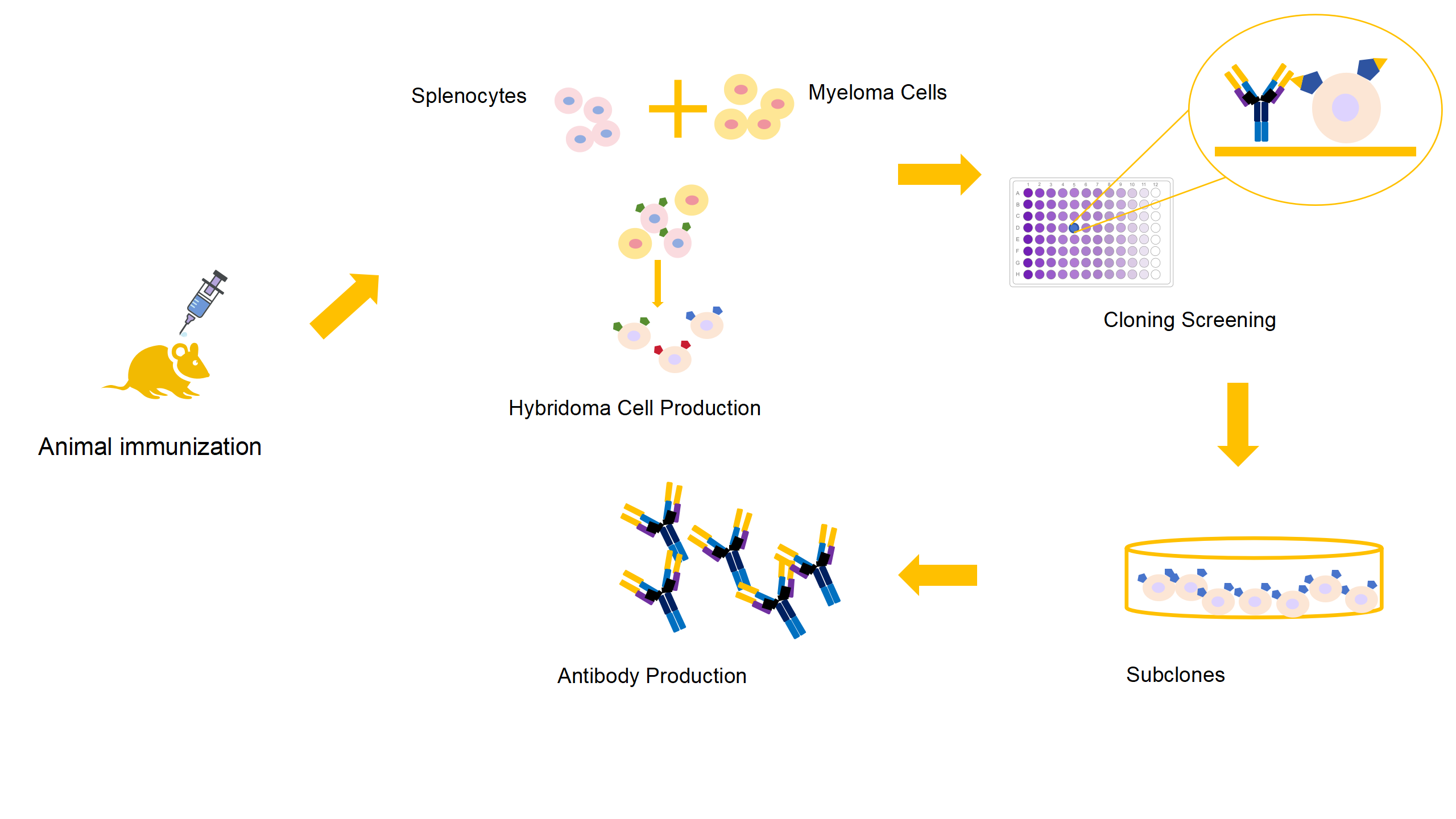
KMD Bioscience offers a complete protocol of Anti-idiotypic antibody production to meet your requirements with our independent Hybridoma Technology Platform. Combined with our proprietary and innovative hybridoma technology and gene synthesis technology, hybridoma cell lines, and peripheral blood lymphocyte samples, KMD Bioscience can provide anti-idotypic antibody production service for our customers (independent clones ~10^13, effective clones ~10^9~10). Monoclonal antibodies with high antigen specificity and high affinity can be screened from these libraries. PCR technology is used to clone the fragment of screened, high-affinity anti-idiotypic antibody to our phagemid expression system. Our systems allow the high expression of anti-idiotypic antibodies in phage, prokaryotic and mammalian expression systems.
Anti-Idiotypic Antibody Production Service Process
Anti-Idiotypic Antibody Application
--Biosimilar antibody immunogenicity study
--Preclinical studies of therapeutic antibodies
--Clinical development of anti-drug antibodies
--Controls in Ligand Neutralization and Antibody Blocking Assays
--Antibody biosimilar pharmacokinetics (PK) and pharmacodynamics (PD) study: Used for high-throughput detection of specific antibody biosimilars including free, binding, and blood.
--We can also provide custom monoclonal antibody development services and custom polyclonal antibody produtcion services, with multiple species, high specificity, and high affinity. For more information, please consult our technical experts.
Anti-Idiotype Antibody Production Service Highlights
--Full traceable support system, GMP/GLP level
--High-throughput antibody screening platform
--High specificity and binding affinity
--Full-service integration
How to Order?
If you have any questions regarding our services or products, please feel free to contact us by E-mail: [email protected] or Tel: +86-400-621-6806