Service Line:+86-022-82164980
Address:FL-4, Building A5, International Enterprise Community, Tianjin, China
Email:[email protected]
KMD Bioscience's monoclonal antibody production is proven and trusted. Monoclonal antibodies are identical immunoglobulins that generated from identical immune cells. Compared to polyclonal antibodies, monoclonal antibodies can be specific to a single epitope and produce large quantities of identical antibodies. For your research, the quality of the antibody affects the outcome of the experiment. That's why KMD Bioscience is committed to providing high-quality monoclonal antibodies and offering customized services to improve your experiments. KMD Bioscience has many successful examples of providing services to Human Monoclonal Antibody Production. The combination of high-quality products and considerate services is our hallmark. There are two methods to produce human monoclonal antibodies: Recombinant monoclonal antibody produced from phage display technology; Hybridoma cell line production by using cell fusion technology. With our upgraded antibody platform, KMD Bioscience provides one-stop services including antigen design, synthesis and modification, in vitro sensitization, phage library construction and screening, antibody modification and application verification.
Human Monoclonal Antibody Production:
After the human body is infected by foreign substances such as viruses, bacteria and parasites, there are lymphocytes in the peripheral blood capable of secreting antibodies against these foreign substances. Based on our extensive experiences in antibody production, KMD Bioscience has developed an independent platform to manufacture human monoclonal antibodies. It is well known that the EBV virus can infect and transform human cells, and the transformed human cells have the characteristics of the proliferation of tumor cells in vitro. At the same time, using peripheral blood mononuclear cells to perform in vitro sensitization, our engineers used isolated and sensitized peripheral blood mononuclear cells infected by the EBV virus, forming a unique sensitized and screened monoclonal cells strategy. This strategy can improve the success rate of antigen specificity and high-affinity human monoclonal antibody production.
Human monoclonal antibody production via our hybridoma-like technology: customers can provide small molecules, peptides, modified peptides, proteins, fusion proteins, modified proteins, etc. as antigens for monoclonal antibody production. Meanwhile, we provide antigen custom services as well, such as DNA synthesis, plasmid construction, peptide synthesis, peptide modification, peptide and carrier protein cross-linking, small molecule structure analysis and carrier protein conjugation.
Service Process:
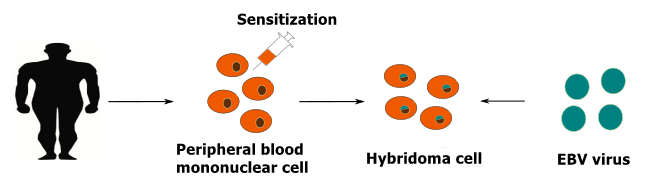
Service Content:
|
Procedure |
Description |
Timeline |
|
Peripheral blood mononuclear cell isolation and in vitro sensitization |
* Collecting peripheral blood from special populations and purifying monocytes
* Culture monocytes in vitro and perform antigen sensitization |
2-3 weeks |
|
Hybridoma Production |
* EBV virus recovery and expansion
* Monocyte immortalization: infection of cell cultures with EBV virus
* Screening and cloning of hybridoma-like cells
* After immunogen detection, select 1-10 positive clones, subcloning and expanded to 96-well plates
* Screening positive subclones by ELISA and expansion of cultivation
* 1mL supernatant or positive clones delivered to investigator for testing |
4-6 weeks |
|
Functional Verification (Optional) |
* ELISA, WB, IF, FLAC, IHC, etc. |
1-3 weeks |
Service Highlights:
--Rich expertise in monoclonal antibody development.
--High affinity and specificity—pM grade.
--Antibodies produced from different sources: rehabilitation and vaccine recipients.
--Antibody humanization, can be used in CAR-T.
--One-stop downstream services, including antigen production, antibody development, ELISA kit development, etc.
How to Order?
If you have any questions regarding our services or products, please feel free to contact us by E-mail: [email protected] or Tel: +86-400-621-6806;