2024-02-18 Hits(166)

Introduction to Phage Display Development Platform
|
KMD Bioscience has achieved excellence in the field of phage presentation of recombinant antibodies and can provide customers with various forms of recombinant antibodies through this technology. Phage display libraries are widely used in the direction of affinity maturation of antibodies, vaccine and antibody drug development by probing highly specific protein-antibody interactions. KMD Bioscience can provide customers with effective phage display solutions with a view to preparing high-quality antibodies, solving experimental challenges and contributing to medical development.
|
|
The Process of Phage Display Antibody Development
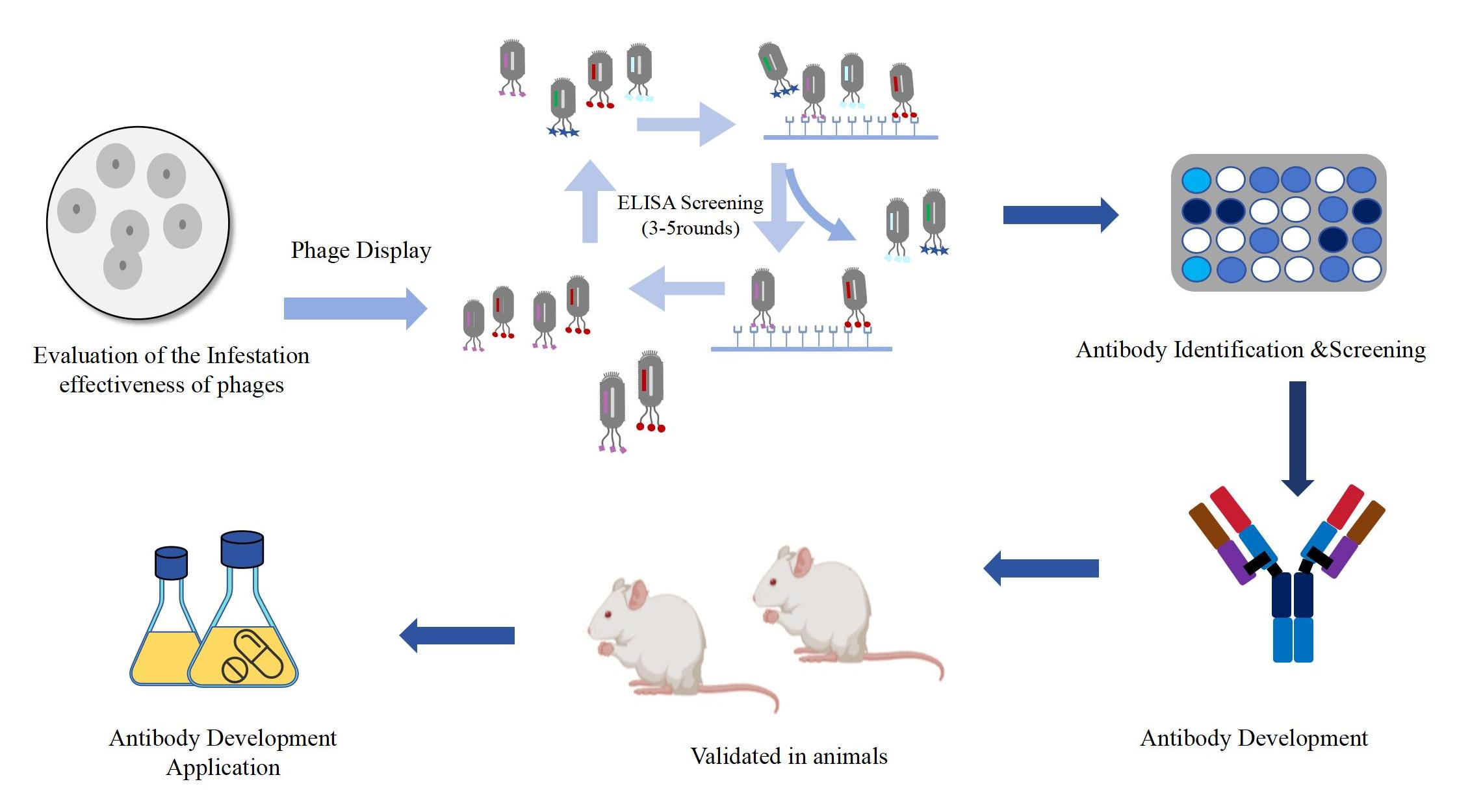
Related Services of Phage Display Antibody Development Platform
Phage display technology utilizes a phage-based display library to identify and develop particular protein and antibody interactions, and is now one of the most potent and widely used laboratory technologies for the study of antibody discovery. KMD Bioscience is devoted to providing one-stop technical services including protein expression, affinity analysis, antibody humanization, antibody affinity maturation and related services to save you time.
|
|
Recommended Special Services
KMD Bioscience can provide customers with phage related services, and its technology platform provides customers with higher quality services.
 |
 |
 |
|
|
|
|
Phage Display Development Platform Related Technical Service Resources
We are willing to share with you the rich technology and resources used in phage display development platform technical services to solve your doubts. If you need to view more, please refer to our related articles.
|
|
|
|
|
|
|
|
|
|
FAQ-Phage Display Antibody Development
Q1: What is the role of phage display library screening in drug discovery and development?
A: Through phage display library screening, researchers can quickly identify high affinity antibodies or peptides that can specifically bind to disease-related proteins or other biomolecules (receptors, enzymes, antigens, etc.). This helps scientists identify new drug targets, develop targeted therapies, and design small molecules that block pathological protein interactions. In addition, phage display library screening can also be used to improve the specificity, reduce side effects, and improve the pharmacokinetic properties of existing drugs. In a word, the phage display library screening strategies accelerate the discovery and preliminary evaluation of new drug candidates and are a powerful tool for modern drug research and development.
Q2: How to ensure the specificity and affinity of antibodies or peptides in the process of phage display library screening?
A: In order to ensure the high specificity and affinity of antibodies or peptides, several experimental procedures are usually adopted:
Multiple rounds of affinity screening: only bacteriophages closely bound to the target were retained in each round of screening.
Competitive elution: competitive elution using excessive free targets ensures that only bacteriophages with high affinity for the target can be selectively retained.
Cross-affinity detection: through affinity testing with other unrelated proteins or targets, the specificity of the screened molecules is evaluated to ensure that they do not cross-react with non-target proteins.
Q3: What are the advantages of phage display library screening compared with other protein screening techniques?
A: High-throughput screening: Billions of different sequences can be processed.
Direct screening of complex samples: Screen target molecules directly from complex biological samples without purifying proteins, greatly simplifying the screening process.
Wide range of applications: Not limited to protein-protein interactions, it can also be used to screen interactions with various biological macromolecules, such as small molecules and nucleic acids.
Functional screening: In addition to affinity and specificity, proteins can also be screened based on their functional activity, providing the possibility to discover new molecules with specific biological functions.
Q4: How to construct a high quality phage display library?
A: Diversity and high coverage: ensure that the display library has a high degree of diversity and can cover a wide range of sequence spaces. This is usually achieved through the use of a variety of coding sequence libraries, including randomized peptide libraries or diversified antibody fragment libraries.
Correct location and expression of insertion sequence: the sequences inserted into the phage genome must be correctly located and effectively expressed to ensure that the target protein or peptide can be correctly folded and displayed on the surface of the phage.
Quality control of the library: through sequence analysis, phage titer determination, and target display efficiency tests to ensure that the phage in the library has a high titer and can effectively display the target sequence.
Avoid repetition: use appropriate strategies to reduce sequence repetition and increase the number of unique sequences in the library, so as to improve the efficiency and success rate of the screening process.
Q5: After phage display library screening, how to verify and make further use of the screened molecules?
A: Affinity and specificity verification: ELISA, surface plasmon resonance (SPR), biological layer interferometer (BLI), and other techniques were used to verify the binding affinity and specificity of the selected molecules to the target.
Functional verification: the biological activity and mechanism of molecules were further verified by functional experiments, such as cell activity determination and signal transduction analysis.
Sequence and structure analysis: sequence analysis and structural modeling of the selected molecules were carried out to understand the molecular basis of their interaction with the target.
Optimization and artificial modification: optimize the selected molecules as needed, such as through artificial evolution, affinity maturation, or engineering modification, to improve their drug properties or production efficiency.
Application development: the screened molecules can be further developed as therapeutic candidates, diagnostic tools, or research reagents.
Q6: Can I use M13 phage display system to construct a cDNA library?
A: Usually, M13 is not suitable for cDNA expression, because the in-frame expression between the leader sequence (required for secretion) and the N-terminus of coat protein pIII or pVIII is required for M13 phage display. In order to fuse the corresponding protein to the coat protein properly, an insert must be in the correct reading frame at both ends and contains no in-frame stop codons. This results in a vanishingly small number of productive clones in M13 cDNA libraries.
Q7: What is the difference between pFu and cFu?
A: The pFu, refers to the plaque-forming unit, is a measure of the quantity of individual infectious particles, and is usually used to count bacteriophage. The cFu, refers to the colony-forming unit, is a measure of viable cells in which a colony represents an aggregate of cells derived from a single progenitor cell, and is usually used to count bacteria (e.g. E. coli).
Q8: Could the helper phage be titrated by blue-white screening?
A: The helper phage provided by KMD Bioscience does not contain lacZ but carry the KanR gene for antibiotic selection, thus cannot be distinguished through blue-white screening.